Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Fe acts a catalyst and Mo acts as promoter in Haber's process.
New answer posted
3 months agoContributor-Level 10
? Solvent is H? O, which is in excess
So using m ( molality ) = (x? *1000)/ (x? * (M? )? )
? x? = 0.74 (Mol? = 18 g)
x? = 1 – 0.74 = 0.26 ∴ m = (0.26 * 1000)/ (0.74 * 18) = 19.5
New answer posted
3 months agoIf Hund's rule is violated, then which among the following will become diamagnetic from paramagnetic
New answer posted
3 months agoContributor-Level 10
BOD value less than 5ppm is considered as clean water and BOD value more than 10ppm is considered as polluted water.
New answer posted
3 months agoContributor-Level 9
Reaction rate is used to measure how fast or slow reactions occur per unit time. The rate constant is a proportionality factor that remains constant for every reaction.
New answer posted
3 months agoContributor-Level 10
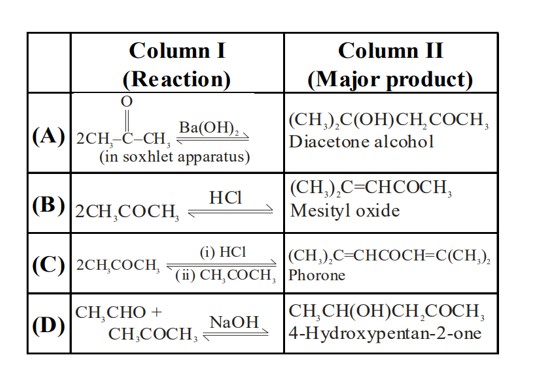
Yield of 4-hydroxypentan-2-one is low because aldol and diacetone alcohol are also formed.
New answer posted
3 months agoContributor-Level 9
Yes, in elementary reactions, order and molecularity can be the same, but this is not always the case because order is an experimental quantity, and molecularity is a theoretical concept.
New answer posted
3 months agoContributor-Level 9
Reaction Kinetics, also known as chemical kinetics, is the study of the rate of chemical reaction and the factors affecting the reaction rate, such as temperature, concentration, and catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers