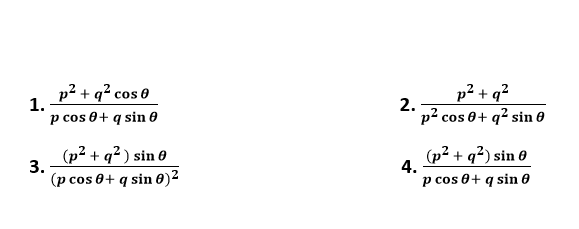JEE Main 2014: Toughest questions of 2013 exam with solutions!
JEE is one of the most sought after engineering exam to get into the top notch engineering institutes across the country. Last year, the exam made its debut on April 7 and for the first time ever, board exams were given huge importance for determining the candidature of applicants.
Due to this, JEE aspirants expected the paper to be complicated. Quite contrary to their expectations, the aspirants were relieved as the paper turned out to be comparatively easy.
According to students, the Physics and Chemistry paper were quite tough but the Mathematics paper was comparatively easy and simple. Overall, JEE Main 2013 was a balanced paper.
Here, check out some of the toughest questions from each of the sections – Physics, Chemistry and Mathematics - in JEE Main 2013:
- Mathematics:
On the whole, the mathematics section of JEE main 2013 was simple. Questions from areas like coordinate geometry, algebra and calculus were within reach. Out of 30 questions, around 18 questions were simple enough to be tackled. However, there were some questions that were quite challenging for the aspirants:

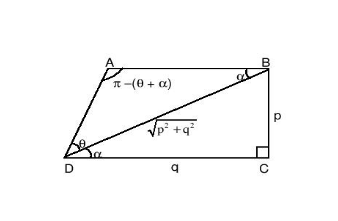
Question 1: ABCD is a trapezium such that AB and CD are parallel and BC is perpendicular to CD. If ∠ADB = θ, BC = p and CD = q, then AB =
Solution: Using the given information we arrive at the following figure:
Now, using the sine rule in ΔABD,
AB/ sin θ = BD/ sin (θ + α)
Hence AB = √(p2 + q2) sin θ/ (sin θ cos α + cos θ sin α) …. (1)
Now, from the figure it is clear that
cos α = q/√(p2 + q2), while sin α = p/√(p2 + q2)
Hence, substituting these values in equation (1) we obtain,
AB = (p2 + q2) sin θ / (p cos θ + q sin θ)
Question 2: If ∫ f(x)dx = Ψ(x), then ∫ x5 f(x3) dx is equal to
1. 1/3 [x3 Ψ (x3) - ∫ x2 Ψ (x3)dx ] + c
2. 1/3 x3 Ψ (x3) – 3 ∫ x3 Ψ (x3)dx + c
3. 1/3 x3 Ψ (x3) –∫ x2 Ψ (x3)dx + c
4. 1/3 [x3 Ψ (x3) - ∫ x3 Ψ (x3)dx ] + c
Solution: It is given that ∫f(x)dx = Ψ(x),
Let I = ∫x5 f(x3) dx
Put x3 = t
Hence, x2 dx = dt/3 …… (1)
So, I = 1/3 ∫ t f(t) dt
Integrating it using by parts we get,
1/3 [t ∫ f(t) dt - ∫ { d(t)/dt ∫ f(t) dt}dt]
= 1/3 [t Ψ(t) - ∫ Ψ(t) dt]
= 1/3 [x3 Ψ (x3) - 3∫ x2 Ψ (x3)dx ] + c (from eq (1))
= 1/3 x3 Ψ (x3) - ∫ x2 Ψ (x3)dx + c
Question 3: A multiple choice examination has five questions. Each question has three alternative answers of which exactly one is correct. The probability that a student will get 4 or more correct answers just by guessing is
1. 17/ 35 2. 13/35
3. 11/35 4. 10/35
Solution: Let us assume the probability of guessing the correct answer to be p
Then the p = 1/3
Hence, the probability of guessing a wrong answer is q = 2/3
Therefore, the probability of guessing 4 or more correct answers is given by
= 5C4 (1/3)4. 2/3 + 5C5(1/3)5
= 5. 2/35 + 1/35
= 11/35.
Hence the correct option is (3).
- Chemistry
The chemistry section in JEE Main 2013 was a perfect blend of average and demanding questions. 14 questions out of the total 30 were quite simple and were based on application of results. The remaining questions were a bit tricky and tested application of the theoretical concepts. Let us have a look at certain daunting questions from this section:
Question 1: An unknown alcohol is treated with the “Lucas Reagent” to determine whether the alcohol is primary, secondary or tertiary. Which alcohol reacts fastest and by what mechanism?
1. tertiary alcohol by SN1 2. secondary alcohol by SN2
3. tertiary alcohol by SN2 4. secondary alcohol by SN1
Solution: The alcohol is treated with the Lucas Regent.
Thus, the reaction proceeds through the carbocation formation. Now, 30 carbocation is highly stable and so the reaction proceeds through SN1 with 30 alcohol. Hence, tertiary alcohol is the fastest to react by SN1 mechanism.
Question 2: How many litres of water must be added to one litre of an aqueous solution of HCl with a pH of 1 to create an aqueous solution with a pH of 2?
1. 09 L 2. 2.0 L
3. 9.0 L 4. O.1 L
Solution: The initial pH of the solution is given to be 1 and the new pH is 2
Initial pH =1 i.e. [H+] = 0.1 mole per litre
New pH =2 i.e. [H+] = 0.01 mole per litre
We know that in case of dilution, M1V1 = M2V2
Therefore, we have 0.1 x 1 = 0.01 x V2
Hence, V2 = 10 L
So, the volume of water added is 9L.
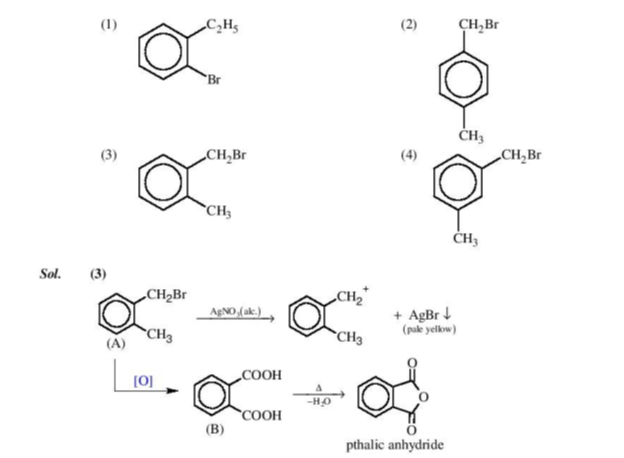
Question 3: Compound (A), C8H9Br gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B), C8H6O4. (B) easily forms an hydride on heating. Identify the compound (A).
- Physics
Students generally have a problem dealing with the Physics section. Infact, out of all the three sections in JEE Mains 2013, physics was the toughest. Out of a total of 30 questions, around 13 questions were of average level. The questions were not only complicated but also demanded lengthy calculations. Let’s look at some of these below:
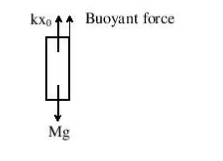
Question 1: A uniform cylinder of length L and mass M having cross-sectional area A is suspended, with its length vertical form a fixed point by a massless spring such that it is half submerged in a liquid of density σ at equilibrium position. The extension x0 of the spring when it is in equilibrium is:
1. Mg/k {1- (LAσ)/ M} 2. Mg/k {1- (LAσ)/ 2M}
3. Mg/k {1 + (LAσ)/ M} 4. Mg/k
(here k is the spring constant)
Solution: We are given a cylinder of length L and mass M having cross-sectional area A. So, we have the following figure
Also, we know that at equilibrium ΣF = 0
So, kx0 + (AL/2. σg) – Mg = 0
Or x0 = Mg [1 – LAσ /2 M]
Hence, the correct option is (2).
Question 2: Assume that a drop of liquid evaporates by a decrease in its surface energy, so that its temperature remains unchanged. What should be the minimum radius of the drop for this to be possible? The surface tension is T, density of liquid is ρ and L is its latent heat of vaporization.
Solution: Let us suppose that the radius of the drop is ‘R’.
Then we know that,
ρ4πR2ΔRL = T4π [R2 – (R – ΔR)2]
ρR2ΔRL = T [R2 - R2 + 2RΔR - Δ R2]
ρR2ΔRL = T2RΔR (ΔR is too small)
Hence, R = 2T/ ρL
The JEE Main 2013 was a balanced paper containing both simple and tricky questions. JEE takers were quite satisfied with the paper as it was easier than the previous years.
Content courtesy: AskIITians.com
Comments
(3)
2014-03-28 20:42:43
2014-03-18 09:59:20
 Call 8585951111
Call 8585951111