
d and f Block NCERT Solutions cover the d-block of the periodic table, which includes the elements of groups 3-12, and the f-block, which includes elements in which 4 f and 5 f orbitals are progressively filled. To refer to the d-and f-blocks, the names transition metals and inner transition metals are used, respectively.
The transition metals in the NCERT Solutions for Class 12 Chemistry Chapter 4 include precious metals like gold, silver, and platinum. It also includes industrially important metals like copper, iron, and titanium.
This chapter explores the occurrence, electronic configuration, and general characteristics of transition elements with a focus on trends in the properties of 3d (first row) of the transition metals. The NCERT solutions are created by experts, and students can rely on these for their exam preparation. It will help them to score high in their CBSE Board exams and other competitive exams like NEET and JEE Main exams.
To get the comprehensive Class 12 Chemistry Notes, which include the topic-wise PDF and solved exercises for quick revision, the students must explore here.
- Key Highlights of d and f block elements Class 12 NCERT Solutions
- Class 12 Chemistry Chapter 4 d and f block Elements: Topics Covered, Weightage
- NCERT Chemistry Class 12 The d and f Block Elements: Important Formulae
- NCERT Solutions Class 12 Chapter 4 d and f-block elements PDF : Download PDF For Free
- Class 12 Chapter 4 The d- and f- block Elements NCERTSolutions: Intext Questions ons
- Benefit of using NCERT Solutions for Class 12 Chemistry Chapter 4
- NCERT Chemistry Chapter 4 The d and f Block Elements – FAQs
Key Highlights of d and f block elements Class 12 NCERT Solutions
Here is a quick summary of the d and f block elements Class 12 Chemistry:
- In the periodic table, the d-block occupies the large middle section. It consists of Groups 3-12.
- Outside the bottom of the periodic table, the f-block is placed, in this part 4f and 5f orbitals are progressively filled.
- Three series of transition elements corresponding to the filling of 3d, 4d, and 5d orbitals are well recognized.
- The transition elements have metallic properties, including ductility, high tensile strength, thermal, malleability, electrical conductivity, and metallic character.
- With increasing atomic number, successive ionisation enthalpies do not increase as steeply as in the main group elements.
- The transition elements vary a lot in their chemical behavior. Also, it reacts with various non-metals like nitrogen, oxygen, halogens, and sulphur to form binary compounds.
- The applications of the d- and f- block elements are found in catalysts, steels, organic syntheses, and complexes.
Related Links
| NCERT Notes for Class 11 & 12 | Class 12 Chemistry NCERT Solutions | NCERT Solutions Class 11 and 12 for Maths, Physics, Chemistry |
Class 12 Chemistry Chapter 4 d and f block Elements: Topics Covered, Weightage
While practicing from the d and f block NCERT solutions, focus on trends in properties like oxidation states, atomic radii, ionization enthalpy, catalytic properties of d-block elements, properties and uses of important compounds like K2Cr2O7 and KMnO4. See below the topics covered in the NCERT Solutions d and f block elements class 12:
| Exercise | Topics Covered |
|---|---|
| 4.1 | Position in the Periodic Table |
| 4.2 | Electronic Configurations of the d-Block Elements |
| 4.3 | General Properties of the Transition Elements (d-Block) |
| 4.4 | Some Important Compounds of Transition Elements |
| 4.5 | The Lanthanoids |
| 4.6 | The Actinoids |
| 4.7 | Some Applications of d- and f- Block Elements |
d and f block elements class 12 Chemistry Chapter 4 Weightage in NEET and JEE Main exam
| Exam | Number of Questions | Weightage |
|---|---|---|
| NEET | 2 questions | 6% |
| JEE Main | few questions | 2.8% |
NCERT Chemistry Class 12 The d and f Block Elements: Important Formulae
Important Concepts and Formulae of Class 12 d and f block elements
- General electronic configuration of d-block elements:
-
-
Cu and Cr are exceptions
-
-
Magnetic moment ( ) depends on unpaired electrons:
where n = number of unpaired electrons.
- f-Block Elements (Inner Transition Elements)
-
Lanthanides (4f-series): General Configuration,
-
Actinides (5f-series): General Configuration,
-
- Uses of Lanthanides:
-
Alloys (Mischmetal used in aircraft engines).
-
Glass polishing & phosphors in TV screens.
-
- Uses of Actinides:
-
U-235 in Nuclear Reactors.
-
Thorium in Breeder Reactors.
-
As per the latest CBSE 2025 Syllabus, class 12 D and F Block Elements chapter has been numbered as chapter 4, earlier it used to be chapter 8.
NCERT Solutions Class 12 Chapter 4 d and f-block elements PDF : Download PDF For Free
Students must download the d and f block NCERT PDF from the link given below. It covers the solutions to all the NCERT textbook questions and helps students understand the concepts clearly. By practicing from this PDF, students can score high in their CBSE Board exam and entrance exams like NEET and JEE Main.
Class 12 Chemistry D and F block Elements NCERT Solution PDF: Download Free PDF
Class 12 Chapter 4 The d- and f- block Elements NCERTSolutions: Intext Questions ons
The d and f block Element Chapter 4 intext questions are based on topics such as transition elements, Lanthanoid contraction, oxidation states, ferromagnetic, Bohr magneton (BM), aqueous solutions, oxides and oxoanions of metal, Inner Transition Elements, lanthanoid contraction, and actinoid contraction. The intext questions are provided after completion of each topic. Solving these questions will help to know how well you have understood the topics. Students must seek the help of a teacher to clear the concept before moving forward. Below are the NCERT Solutions for d and f block elements intext questions.
| Q 8.1 Silver atom has completely filled d orbitals (4d 10) in its ground state. How can you say that it is a transition element? |
| Ans The elements which have partially filled d or f subshells in any common oxidation state are called as the transition elements. Silver (the Atomic number is 47 and electronic configuration is [Kr] 4d105s1) has a completely filled 4d orbital in its ground state but has two oxidation states (+1, +2). In the +1 oxidation state, an electron is removed from the s-orbital and in +2 oxidation state, one electron from d-orbital is also removed. Thus, the d-orbital now becomes partially filed (4d9). Hence, it is a transition element. |
| Q 8.2 In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest, i.e., 126 kJ mol–1. Why? |
| Ans The enthalpy of atomization depends on the strength of the metallic bonding. Stronger the metallic bonding, greater is the enthalpy of atomization. The metallic bonding is strong when there are more unpaired electrons in the atom. All transition metals (except Zn, electronic configuration: [Kr] 3d10 4s2), have at least one unpaired electron that is responsible for their stronger metallic bonding. Since the Zn atom does not have an unpaired electron, the metallic bonding is weak and hence the enthalpy of atomization is low. |
| Q 8.3 Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why? |
| Ans Manganese (Z = 25) exhibits the largest number of oxidation states. This is because its electronic configuration is 3d54s2. Because it has the maximum number of electrons (5 d electrons and 2s electrons) to easily lose and share. Therefore, it can exhibit an oxidation state of +2 to +7. The compounds are as follows: Mn(0) as Mn(s), Mn(II) as MnO, Mn(II,III) , Mn etc. |
| Q 8.4 The E V (M2+/M) value for copper is positive (+0.34V). What is possible reason for this? (Hint: consider its high ∆aH V and low ∆hydH V ) |
| Ans The E0(M2+/M) value of a metal depends on the energy changes involved in the following reactions: 1. Sublimation energy: The energy needed to convert one mole of atoms from a solid state to gaseous 2. Ionization energy: The energy supplied to remove electrons from one mole of atoms, which are in the gaseous 3. Hydration energy: The energy emitted to hydrate one mole of Now, copper has a high ionisation energy and low hydration energy. Hence, the E0(M2+/M) value for copper is positive. |
Commonly asked questions
8.27 For M2+/M and M3+/M2+ systems the E V values for some metals are as follows: Cr 2+/Cr - 0.9V Cr 3 /Cr 2+ -0.4 V Mn2+/Mn -1.2V Mn3+/Mn2+ +1.5 V Fe2+/Fe -0.4V Fe3+/Fe2+ +0.8 V Use this data to comment upon:
(i) The stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+
(ii) The ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
8.27 (i) Reduction potential tells us the ease with which the Metal can get reduced, As E° for Cr3+/Cr2+ is negative (–0.4 V), this means that Cr3+ ions in solution cannot be reduced to Cr2+ ions i.e., Cr3+ ions are very stable. As a further comparison of E° values show that Mn3+ ions more readily than Fe3+ ions which means that Mn3+ is least stable.
So Stability of metal ions is as follows:
Mn3+
(ii) Reduction potential tells us the ease with which the Metal can get reduced or the difficulty with which they are oxidized, As the reduction potential increases in the following order Mn2+/Mn < Cr2+/Cr< Fe2+/Fe
So oxidation of Fe is not as easy as Cr and Mn.
So increasing order of the metals to get oxidised is as follows:
Fe < Cr < Mn
8.37 What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
8.37 An alloy is a homogenous mixture of two or more metals or non-metals. Lanthanoid is used for the production of alloy steels for plates and pipes called as mischmetals. It contains about 95% lanthanoid metal, iron and traces of Al, S, C etc. in traces. It is used in magnesium- based alloy to produce bullets, shells and lighter flint.
8.21 Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
(ii) The enthalpies of atomisation of the transition metals are high.
(iii) The transition metals generally form coloured compounds.
(iv) Transition metals and their many compounds act as good catalyst.
8.21 (i) Transition metals and many of their compounds show paramagnetic behavior- Paramagnetic behaviour is shown by transition metals as paramagnetism is due to the presence of unpaired electrons which have a magnetic moment associated with its spin and angular momentum, as the orbital angular momentum is satisfied in the first transition series. So the paramagnetic is only due to the unpaired electrons.
(ii) The enthalpies of atomisation of the transition metals are high- Enthalpies of atomization is the enthalpy change when 1 mol of gaseous atoms is formed from its element in its defined physical state under standard conditions (298.15K, 1 atm). In case of the transition metals enthalpies are high due to high effective nuclear charge and a large number of valence electrons which also leads to strong metallic bonding.
Eg: 515 KJ/mol for Vanadium,473 KJ/mol for Titanium
(iii) The transition metals generally form coloured compounds- Transition metals are generally coloured because of absorption of radiations which fall in the visible region as on obtaining energy electrons jump from one d-orbital to another.
(iv) Transition metals and their many compounds act as good catalyst- Transition metals and their compounds act as good catalysts due to its ability to show variable oxidation state and form complexes, another reason is that they provide a suitable surface for reaction to take place.
Eg: Vanadium Oxide in contact process, Finely divided iron in Haber's Process.
8.7 Which is a stronger reducing agent Cr2+ or Fe2+ and why ?
8.7 The following reactions are involved when Cr2+ and Fe2+ act as reducing
Cr2+ = Cr3+ ( E? Cr3+/ Cr2+ = - 0.41 V)
Fe2+⇒ Fe3+ (E? Fe3+/ Fe2+ = +0.77 V)
Since, Cr has less potential value, Cr2+ gets oxidised easily than Fe2+. Therefore, Cr2+ is a better reducing agent that Fe3+.
8.6 Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
8.6 The oxidation state increases when an atom loses its Example: When Fe loses 2 electrons, its oxidation state becomes +2 from 0.
Oxygen (O) and fluorine (F) are very strong oxidizing agents. Both oxide and fluoride ions are highly electronegative and have a very small size, so they attract the electrons from metal atoms. Hence, they oxidize the metal to its the highest oxidation state.
8.9 Explain why Cu+ ion is not stable in aqueous solutions?
8.9 The stability in aqueous condition depends on the hydration energy of the ions when they bond to the water molecules. And, the hydration energy is the amount of heat released as an ionic substance is dissolved and its constituent ions are hydrated or surrounded by water molecules.
Now, in Cu2+ and Cu+ ion, Cu2+ has a greater charge density than the Cu+ ion and so forms much stronger bonds releasing more energy. Therefore, in an aqueous medium, Cu2+ ion is more stable than Cu+ ion. This is because the energy required to remove one electron from Cu+ to Cu2+, is compensated by the high hydration energy of Cu2+.
8.5 How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
8.5 The ionization energy increases due to the gradual filling of electrons in the d- subshells. The irregular variation of ionization energy is due to the fact that half-filled and completely filled subshells are more stable and have very high ionization energy.
In case of first ionization energy, Cr ( [Ar]3d54s1) attains the stable configuration (3d5) by losing one electron from s-subshell and hence, it has low ionization energy.
Whereas, Zn has high ionization energy because it has completely filled subshells and are very stable.
Second ionization energies are higher than the first since it becomes difficult to remove an electron when an electron has to be removed from a stable structure, which was formed due to the removal of the first electron.
8.8 Calculate the ‘spin only’ magnetic moment of M2+(aq) ion (Z = 27).
8.8 Given, Z=atomic number = 27 = [Ar]3d7 4s2
⇒ M2+ = [Ar]3d7
Hence, 3 unpaired electrons are present.
The spin only magnetic moment μ= √n(n+2)
Where n is the number of unpaired electrons.
Hence, μ =√(3×(3+2))
μ = √15 BM or μ = 3.87 BM
8.48 What can be inferred from the magnetic moment values of the following complex species ?
|
Example |
Magnetic moment |
|
[K4 [Mn(CN)6] |
2.2 |
|
[Fe(H2O)6]2+. |
5.3 |
|
K2 [MnCl4] |
5.9 |
8.48 To calculate magnetic moment of the complex species, we use the spin formula:
μ =√n(n+2) BM
When n= 1 | ⇒ μ = √1(1+2) ⇒ u= √3⇒ u=1.73 BM |
When n=2 | ⇒ μ = √2(2+2) ⇒ μ = √8 ⇒ μ = 2.83 BM |
When n= 3 | ⇒ μ = √3(3+2) ⇒ μ = 15 ⇒ μ = 3.87 BM |
When n = 4 | ⇒ μ = √ 4(4+2) ⇒ μ = √24 ⇒ μ = 4.899 BM |
When n= 5 | ⇒ μ = √5(5+2) ⇒ μ = √35 ⇒ μ = 5.92 BM |
1. [K4 [Mn(CN)6]
⇒μ = 2.2 BM (given)

We can see from the above calculation that the given value(2.2) is close to n=1. It means that it has only one unpaired electron Also in this complex Mn is in +2 oxidation state,i.e., as Mn2+.Thus when CN- ligands approach Mn2+ ion, The electrons in 3d do not pair up.
The atomic number of Manganese (Mn) is Z = 25
The electronic configuration of 25Mn= [Ar] 3d5 4s2
And, the electronic configuration of Mn2+=[Ar] 3d5
Thus CN- is a strong ligand.
The hybridization involved is d2sp3 forming inner orbital octahedral complex
2. [Fe(H2O)6]2+.
⇒ μ = 5.3 BM (given)
We can see from the above calculation that the given value(5.3) is close to n = 4. It means that it has four unpaired electrons. In this complex, Fe is in +2 state, i.e., as Fe2+. This means that the electrons in 3d do not pair up when the ligands, H2O molecules approach.
The atomic number of Iron(Fe)is Z = 26
The electronic configuration of 26Fe= [Ar] 3d6 4s2
And, the electronic configuration of Fe2+=[Ar] 3d6
Thus H2O is a weak ligand. To accommodate the electrons donated by six H2O molecules, the hybridization will be sp3d2. Hence it will be an outer orbital octahedral complex.
3. K2 [MnCl4]
⇒μ = 5.9 BM (given)
We can see from the above calculation that the given value(5.9) is close to n=5. It means that it has five unpaired electrons
In this complex, Mn is in +2 state, i.e., as Mn2+. Hence, we can say that Cl- is a weak ligand and does not cause the pairing of electrons.

The atomic number of Manganese (Mn) is Z = 25
The electronic configuration of 25Mn= [Ar] 3d5 4s2
And, the electronic configuration of Mn2+=[Ar] 3d5
Thus, the hybridization involved will be sp3 and the complex will be tetrahedral complex
Being Lewis bases (those who donate electrons) the ligands with less electronegativity will be stronger. Therefore, in general halogen or oxygen donors (F-, Cl-,Br-, H2O) are weak field ligands and the ones in which carbon or nitrogen atom is the donor (eg-CN-,CO,NH3) are strong field ligands.
8.1 Silver atom has completely filled d orbitals (4d 10) in its ground state. How can you say that it is a transition element?
8.1 The elements which have partially filled d or f subshells in any common oxidation state are called as the transition elements. Silver (the Atomic number is 47 and electronic configuration is [Kr] 4d105s1) has a completely filled 4d orbital in its ground state but has two oxidation states (+1, +2).
In the +1 oxidation state, an electron is removed from the s-orbital and in +2 oxidation state, one electron from d-orbital is also removed. Thus, the d-orbital now becomes partially filed (4d9). Hence, it is a transition element.
8.3 Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
8.3 Manganese (Z = 25) exhibits the largest number of oxidation states. This is because its electronic configuration is 3d54s2.
Because it has the maximum number of electrons (5 d electrons and 2s electrons) to easily lose and share.
Therefore, it can exhibit an oxidation state of +2 to +7. The compounds are as follows:
Mn (0) as Mn (s), Mn (II) as MnO, Mn (II, III), Mn etc.
8.45 Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
(i) Electronic configurations
(ii) Oxidation states
(iii) Ionisation enthalpies
(iv) Atomic sizes.
8.45 (i) Electronic configuration:
In the first transition series, 3d orbitals are progressively filled while in the second and third transition series, 4d and 5d orbitals are filled. However the first series shows only two exceptions Cr and Cu, both have a single electron in the 4s orbital ( 3d5 4s1, 3d10 4s1) but the second series shows more exceptions. Similarly, third series elements show exceptions. Thus in the same vertical column, in a number of series, the electronic configuration of the three series are not similar at all.
(ii) Oxidation states:
The number of oxidation states shown by the elements in the middle of each series is maximum and minimum at the extreme ends. However, the first row elements differ from the second and third-row elements in the fact that for all the first row elements, +2 and +3 states are important. For second and third row elements, higher oxidation states are more important than those of the first row elements.
(iii) Ionisation enthalpies:
The first ionization enthalpies in each series generally increase gradually as we move from left to right through some exceptions are observed in each series. The first ionization enthalpies of some elements in the second (4d) series are higher while some of them have a lower value than the elements of 3d series in the same vertical column.
However the first ionization enthalpies of third (5d) series are higher than those of 3d and 4d series. This is because of weak shielding of nucleus by 4f electrons in the 5d series
(iv) Atomic sizes:
In general, ions of the same charge or atoms in a given series show progressively decrease in radius with increasing atomic number though the decrease is quite small. But the size of the atoms of the 4d series is larger than the corresponding elements of the 3d series whereas those of corresponding elements of the 5d series are nearly the same as those of4d series because of Lanthanoid contraction
Note: Lanthanoid contraction is the regular decrease (contraction) in the atomic and ionic radii of lanthanoids with increasing atomic number
8.43 Compare the chemistry of the actinoids with that of lanthanoids with reference to: (i) electronic configuration (ii) oxidation states and (iii) chemical reactivity.
8.43
Lanthanoids |
Actinoids |
1.The electronic configuration of lanthanoids is [Xe]54 4f1-14 | 1.The electronic configuration of actinoids is [Rn]86 5f1-14 6d0-1 7s2 |
2. They show limited oxidation states (+2, +3, 4) out of which +3 is most common. This is because of the large energy gap between 4f and 5d subshells. | 2. Actinoids show a large number of oxidation states (+3, +4, +5, +6, +7) because of the small energy gap between 5f, 6d, and 7s subshells. |
3. The first few members of the series are quite reactive, almost like calcium with increasing atomic no. their behaviour becomes similar to that of aluminium | 3.They are highly reactive metals especially in the finely divided state. When they are added to boiling water they give a mixture of oxide and hydride. |
8.35 Give examples and suggest reasons for the following features of the transition metal chemistry:
(i) The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
(ii) A transition metal exhibits highest oxidation state in oxides and fluorides.
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
8.35 (i) The lower oxide have low oxidation state while the higher oxide has high oxidation state, example MnO is basic and Mn2O7 is
(ii) Oxygen and fluorine have a small size and high electronegativity and can easily oxidize metals, example V2O5.
(iii) Oxoanions of metals have higher oxidation states because of high electronegativity of oxygen and highly oxidizing property example, Cr in CrO7 2- has an oxidation state of +6
8.25 Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with: (i) iodide (ii) iron(II) solution and (iii) H2S
8.25 K2Cr2O7 acts as a very strong oxidizing agent in acidic medium. K2Cr2O7 gets reduced and acts as an oxidizing agent by oxidizing Iodide to iodine
(i) K2Cr2O7 oxidizes iodide to iodine
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
2I-→ I2 + 2e- } x3
Overall: Cr2O72-+ 14H++ 6I-→ 2Cr3+ + 7H2O+ 3I2
(ii) K2Cr2O7 oxidizes iron(II) to iron(III)
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
Fe2+→ Fe3+ + e- } x6
Overall: Cr2O72-+ 14H++ 6Fe2+→ 2Cr3+ + 7H2O + 6Fe3+
(iii) K2Cr2O7 oxidized H2S to sulphur
Cr2O72-+ 14H++ 6e-→ 2Cr3+ + 7H2O
H2S → S + 2H+ + 2e-} x3
Overall: Cr2O72-+ 8H++ 3H2S → 2Cr3+ + 7H2O +3S
8.10 Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
8.10 The 5f orbitals (in actinoids) have a poorer shielding effect than 4f orbitals (in lanthanoids). Thus, the effective nuclear charge experienced by electrons in outer shells in case of actinoids is much more that experienced by lanthanoids.
As the effective nuclear charge experienced is high, the electrons are attracted with much force, hence the size of the atom decreases. Hence, actinoid contraction is greater from element to element than lanthanoid contraction.
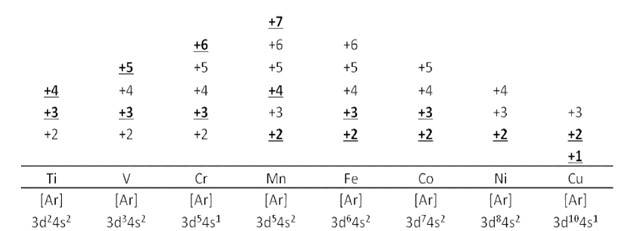
8.13 Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
8.13 The oxidation states displayed by the first half of the first row of transition metals are given in the table below.
METALS | Sc ( [Ar] 3d14s2) | Ti ( [Ar] 3d24s2) | V ( [Ar] 3d34s2 ) | Cr ( [Ar] 3d54s1) | Mn ( [Ar] 3d54s2) |
OXIDATION STATES |
| +2 | +2 | +2 | +2 |
+3 | +3 | +3 | +3 | +3 | |
| +4 | +4 | +4 | +4 | |
|
| +5 | +5 | +6 | |
|
|
| +6 | +7 |
Except for Sc, all others metals display +2 oxidation state. This is because as the atomic number increases, the number of electrons in the valence shell increases. +2 oxidation state is attained by the loss of the two 4s electrons by these metals. As the number of electron increases, the possibility of an ion with +2 oxidation state being stable (by attaining half-filled structure) also increases. Finally, Mn2+ ions have half-filled structure and are very stable.
8.11 Write down the electronic configuration of: (i) Cr3+ (iii) Cu+ (v) Co2+ (vii) Mn2+ (ii) Pm3+ (iv) Ce4+ (vi) Lu2+ (viii) Th4+
8.11 (i) The atomic number of Cr is 24 and the electronic configuration is [Ar] 3d54s1. When 3 electrons are removed, it becomes Cr3+. The electronic configuration of Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3 Or [Ar]3d3
(ii) The atomic number of Pm is 61 and the electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f5 5s2 5p6 6s2 or [Xe] 4f56s2. When 3 electrons are removed, it becomes Pm+3, having the electronic configuration,
Pm3+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4
Or, [Xe]544f4
(iii) The atomic number of Cu is 29 and has the electronic configuration of [Ar] 3d104s1. On removing one electron, the Cu+is obtained with the electronic configuration, 1s2 2s2 2p6 3s2 3p6 3d10 Or [Ar] 3d10
(iv) The atomic number of Ce is 58 and has the electronic configuration of [Xe] 4f15d16s2. When the valence electrons are removed, the Ce4+ ion with configuration, 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 Or [Xe] is obtained.
(v) The atomic number of Co is 27 and has the electronic configuration of [Ar] 3d74s2. When 2 electrons are removed from s-orbital, it becomes Co2+with electronic configuration, 1s2 2s2 2p6 3s2 3p6 3d7 Or, [Ar] 3d7.
(vi) The atomic number of Lu is 71 and has the electronic configuration of [Xe] 4f145d16s2. When 2 electrons are removed, Lu2+with the configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f 14 5d 1 Or, [Xe] 4f145d1
(vii) The atomic number of Mn is 25 and has the electronic configuration of [Ar] 3d54s2 . When 2 electrons are removed, Mn2+ ion is obtained and has an electronic configuration of 1s2 2s2 2p6 3s2 3p6 3d10 Or, [Ar]18 3d10 .
(viii) The atomic number of Th is 90 and has the electronic configuration of [Rn] 6d2 7s2 . When 4 electrons are removed, the electronic configuration becomesTh4+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f 14 5d 10 6s 2 6p 6 Or, [Rn]
8.20 What are the different oxidation states exhibited by the lanthanoids?
8.20 +3 oxidation state is the most common oxidation state of lanthanides i.e., Ln (III) compounds are predominant. +2 and +4 oxidation states can also be found in the solution or in solid compounds, but are not predominant.
8.16 Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
8.16 (i) Vanadate (VO3 -)-Oxidation state of V is +
(ii) Chromate (CrO4 2-)-Oxidation state of Cr is +
(iii) Permanganate (MnO4 -) Oxidation state of Mn is + 7
8.22 What are interstitial compounds? Why are such compounds well known for transition metals?
8.22 Interstitial compounds are those compounds in which small atomic size elements like H, C, N occupy the interstitial sites of the crystal lattice of the Transition metal due to their large size.
Example, in the diagram given below:
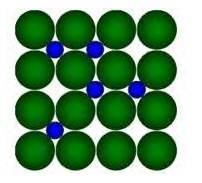
Here, Green Balls form the Crystal lattice of transition metal and Blue balls represent elements like H/C/N which occupy the interstitial sites.
8.31 How would you account for the following:
(i) Of the d 4 species, Cr2+ is strongly reducing while manganese(III) is strongly oxidising.
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
(iii) The d 1 configuration is very unstable in ions.
8.31 (i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidizing: The +2 oxidation state becomes more stable on moving across a period i.e. the tendency of metals to give electrons becomes more. Therefore, vanadium(II) oxide and chromium(II) oxide are strong reducing agents.
As if the value of electrode potential is higher i.e. more energy required to withdraw an electron from an isolated atom, more readily it can be reduced and lesser the electrode potential more readily it can be oxidized.
The electrode potential of Cr3+? Cr2+is negative so it acts reducing agent or can undergo oxidation which makes it a more stable ion while that of Mn3+? Mn2+ is positive and it undergoes reduction acts as strong oxidizing agent. Also, Mn3+ has an exactly half-filled d- orbital which is highly stable.
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidized: Cobalt gets oxidized from +2 to +3 oxidation state in the presence of complexing reagents.
(iii) The d1 configuration is very unstable in ions:The d1 electron can be easily lost after the loss of ns electrons. Therefore, the elements having d1 configuration undergo disproportionation reactions.
8.30 Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(i) Electronic configuration
(ii) Atomic and ionic sizes
(iii) Oxidation state
(iv) Chemical reactivity.
8.30
| Lanthanoids | Actinoids |
Electronic Configuration | It is represented by [Xe]4fx5dy6s2, where x varies from 0 to 14 and y= 0 or 1 | It is represented by [Rn]5fx6dy7s2, where x varies from 0 to 14 and y= 0 or 1. |
Oxidation state | Generally shows +3 oxidation state only in some cases it is +2 or +4 but never greater than +4 | It has +3 oxidation state but also shows higher oxidation states such as +4, +5, +6, +7. |
Atomic and ionic sizes | Ionic radii of M3+ions decrease in size with increase in atomic number this is called as lanthanoid contraction. | There is a gradual decrease in the size of M3+ ions across the series, this is known as actinoid contraction. |
Chemical Reactivity | Lanthanoids are less reactive in nature and form oxides, sulphides, nitrides etc. They have a lesser tendency to form complexes. | They are highly reactive in nature when they are in the finely divided state. They have a higher tendency to form complexes and even react with non-metals at moderate temperature. |
Actinoid Contraction > Lanthanoid contraction
Lanthanoids show lanthanoid contraction due to which their size is quite small as compared to actinoids although there is actinoid contraction also lanthanoid contraction has more impact on elements as there is one shell less than actinoids, so lanthanoids have less tendency to lose an electron and to undergo any reaction like the formation of oxide etc.
8.12 Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
8.12 Electronic configuration of Mn2+ is [Ar]183d5 and Electronic configuration of Fe2+ is [Ar]18 3d6 . It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in (+2) state has a half-filled stable configuration, whereas the Fe in +3 oxidation state has partially filled subshells, which are relatively unstable. This is the reason Mn2+ shows resistance to oxidation to Mn3+. Also, Fe2+ has 3d6 configuration and by losing one electron, it attains half- filled stable Hence, Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state.
Mn+2→
Manganese has the atomic no. 25 and its electronic configuration is [Ar]4s23d5.
∴ Electronic configuration of Mn+2=[Ar]3d5
Fe+2→
Fe has the atomic no. 26 and its electronic configuration is [Ar]4s23d6.
∴ Electronic configuration of Fe+2=[Ar]3d6
8.15 What may be the stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms : 3d3 , 3d5 , 3d8 and 3d4 ?
8.15 For answering this question, we can compare the electronic configuration of standard elements and then write their corresponding oxidation
S. No | Electronic configurations in ground state | Stable oxidation states |
1 | 3d3 Vanadium | +2, +3, +4, +5 |
2 | 3d5 Chromium | +3, +4, +6 |
3 | 3d5 Manganese | +2, +4, +6, +7 |
4 | 3d8 Nickel | +1, +2, +3, +4 |
5 | 3d4 | 3d4 configuration is not stable at ground state |
8.4 The E V (M2+/M) value for copper is positive (+0.34V). What is possible reason for this? (Hint: consider its high ?aH V and low ?hydH V )
8.4 The E0 (M2+/M) value of a metal depends on the energy changes involved in the following reactions:
1. Sublimation energy: The energy needed to convert one mole of atoms from a solid state to gaseous
2. Ionization energy: The energy supplied to remove electrons from one mole of atoms, which are in the gaseous
3. Hydration energy: The energy emitted to hydrate one mole of
Now, copper has a high ionisation energy and low hydration energy. Hence, the E0 (M2+/M) value for copper is positive.
8.14 To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
8.14 The elements in the first-half of the transition series exhibit many oxidation states with Mn exhibiting a maximum number of oxidation states (+2 to +7). The stability of +2 oxidation state increases with the increase in atomic number. This happens as more electrons are getting filled in the d-orbital.
However, Sc ( [Ar] 3d14s2) does not show +2 oxidation state, instead, it loses all the three valence electrons to form Sc3+. The +3 oxidation state of Sc is very stable as it attains stable configuration.
For Mn ( [Ar] 3d54s2), +2 oxidation state is very stable because after losing two electrons, it attains stable half-filled structure.
8.38 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
8.38 The inner transition elements are those in which the last electron enters the f-subshell. The lanthanoids atomic number 58-71 and actinoids 90-103. The atomic numbers 59, 95 and 102 are inner transition elements
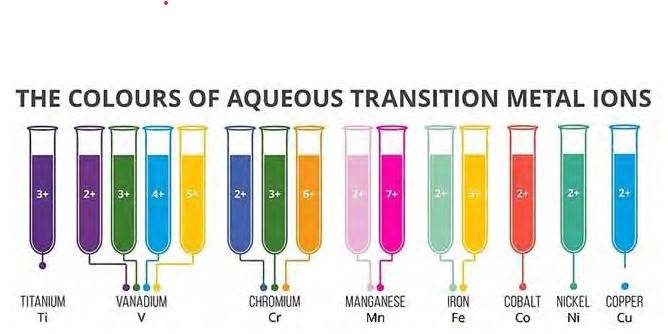
8.28 Predict which of the following will be coloured in aqueous solution? Ti3+, V3+ , Cu+ , Sc 3+, Mn2+, Fe 3+ and Co 2+. Give reasons for each.
8.28 Metal ions which have valence electrons in d-orbital and in which d-d transition can take place will be coloured and the metal ions which have completely filled orbital or have d- orbital will be colourless as no d-d transition is possible in those configurations.
Element | Atomic Number | Ionic State | Electronic configuration in ionic state |
Ti | 22 | Ti3+ | [Ar] 3d1 |
V | 23 | V3+ | [Ar] 3d2 |
Cu | 29 | Cu+ | [Ar] 3d10 |
Sc | 21 | Sc3+ | [Ar] |
Mn | 25 | Mn2+ | [Ar] 3d5 |
Fe | 26 | Fe3+ | [Ar] 3d5 |
Co | 27 | Co2+ | [Ar] 3d7 |
From the above table, it can be observed that only Sc3+ and Cu+ have either completely filled d- orbital or empty d-orbital. So, all other ions except Sc3+ and Cu+ will be coloured in aqueous solution because of absorption of radiations which fall in the visible region as on obtaining energy electrons jump from one d-orbital to another.
8.36 Indicate the steps in the preparation of: (i) K2Cr2O7 from chromite ore. (ii) KMnO4 from pyrolusite ore.
8.36 (i) The chromite ore (FeCr2O4) is fused with sodium hydroxide (NaOH)
4FeCr2O4 +16 NaOH+ 7O2 → 8Na2CrO4 +2Fe2O3 +8H2O
The yellow solution of sodium chromate is then filtered and acidified with sulphuric acid giving its dichromate.
2Na2CrO4 +H2SO4→ Na2Cr2O7 + Na2SO4 +H2O
On cooling, sodium sulphate crystallizes out as Na2SO4.10H2O and is removed.
Na2Cr2O7 +2KCl → K2Cr2O7 + 2NaCl
(ii) The pyrolusite ore(MnO2) is oxidised in the presence of potassium hydroxide by heating.
2MnO2 + 4KOH +O2→ 2K2MnO4 + 2H2O
The green potassium manganate (K2MnO4) is then treated with a current of chlorine or ozone to oxidise potassium manganate to potassium permanganate.
2K2MnO4 +Cl2→ 2KCl +2KMnO4
K2MnO4 +O3 +H2O → 2KMnO4 +2KOH +O2
8.34 Calculate the number of unpaired electrons in the following gaseous ions: Mn3+ , Cr3+, V3+ and Ti3+. Which one of these is the most stable in aqueous solution?
8.34 From the table given below:
Mn3+ : 3d4 | unpaired electrons = 4 |
V3+ : 3d2 | unpaired electrons =2 |
Cr3+ : 3d3 | unpaired electrons= 3 |
Ti3+ : 3d1 | unpaired electrons =1 |
Cr3+ is most stable in aqueous solution because of half filled d-orbital.
8.39 The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
8.39 Lanthanoids show oxidation states of +2, +3, +4 because of the large energy gap between 5d and 4f sub-shells while actinoids show oxidation states of +3 to +7 because of the small energy difference between 5f, 6d, and 7s orbitals.
8.24 Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
8.24 Potassium dichromate is prepared from chromite ore (FeCr2O4) by the following steps:
Step1: Preparation of sodium dichromate in the reaction of Chromite ore with sodium hydroxide and oxygen gas.
4FeCr2O4 + 16NaOH + 7O2? 8Na2CrO4 + 2Fe2O3 + 8H2O
Step2: Conversion of Sodium Chromate on reaction with concentrated Sulfuric acid gives Sodium dichromate as a product.
2Na2CrO4 + conc. H2SO4? Na2Cr2O7 + Na2SO4 + H2O
Step3: Sodium dichromate on reaction with potassium chloride converts to potassium dichromate as a product.
Na2Cr2O7 + 2KCl? K2Cr2O7 + 2NaCl
As potassium dichromate is less soluble than sodium chloride so, potassium dichromate is obtained in form of orange crystals.
Dichromate ion exists in equilibrium with chromate ion at around pH. However, by changing the pH they can be interconverted.
8.42 Name the members of the lanthanoid series which exhibit +4 oxidation states and those which exhibit +2 oxidation states. Try to correlate this type of behaviour with the electronic configurations of these elements.
8.42 The members of lanthanide series along with their electronic configuration are given in the below table:
Name | Symbol | Atomic number | Electron configuration |
lanthanum | La | 57 | (Xe)5d1 6s2 |
Cerium | Ce | 58 | (Xe)4f15d06s2 |
Praseodymium | Pr | 59 | (Xe)4f35d06s2 |
Neodymium | Nd | 60 | (Xe)4f45d06s2 |
Promethium | Pm | 61 | (Xe)4f55d06s2 |
Samarium | Sm | 62 | (Xe)4f65d06s2 |
Europium | Eu | 63 | (Xe)4f75d06s2 |
Gadolinium | Gd | 64 | (Xe)4f7 5d16s2 |
Terbium | Tb | 65 | (Xe)4f95d06s2 |
Dysprosium | Dy | 66 | (Xe)4f105d06s2 |
Holmium | Ho | 67 | (Xe)4f115d06s2 |
Erbium | Er | 68 | (Xe)4f125d06s2 |
Thulium | Tm | 69 | (Xe)4f135d06s2 |
Ytterbium | Yb | 70 | (Xe)4f145d06s2 |
Lutetium | Lu | 71 | (Xe)4f14 5d16s2 |
The typical oxidation state of the lanthanides is +3. The oxidation state of +2 and +4 are exhibited by some of the elements. These are shown by those elements which by losing 2 or 4 elements acquire a stable configuration.
+2 oxidation state is exhibited when the lanthanide has the configuration 5d06s2 so that 2 electrons are lost easily. Hence the members which will show +2 oxidation state are:
+2 = 60Nd, 62Sm, 63Eu, 69Tm, 70Yb
+4 oxidation state is exhibited when the configuration left (by losing 2 electrons) is close to 4f0 (example 4f0 , 4f1, 4f2, 4f3) or close to 4f7 (example 4f7or 4f8) or close to 4f14 ( 4f13 or 4f14)
Hence, the members which will show +4 oxidation state are:
+4 = 58Ce, 59Pr, 60Nd, 65Tb, 66Dy
Each case tends to revert to the more stable oxidation state of +3 by loss or gain of an That is why Sm2+, Eu2+ and Yb2+ ions in solutions are good reducing agents and aqueous solution of Ce4+ and Tb4+ are good oxidizing agents.
8.44 Write the electronic configurations of the elements with the atomic numbers 61, 91, 101, and 109.
8.44 Promethium (Pm) has atomic number 61. Hence electronic configuration of Promethium is [Xe]544f5 5d0 6s2
Protactium (Pa) has atomic number 91. Hence electronic configuration of Protactium is [Rn]86 5f2 6d1 7s2
Mendelevium (Md) has atomic number 101. Hence electronic configuration of Mendelevium is [Rn]86 5f14 6d0 7s2
Meitnerium (Mt) has atomic number 109. Hence electronic configuration of Meitnerium is [Rn]86 5f14 6d7 7s2
8.18 What are the characteristics of the transition elements and why are they called transition elements? Which of the d-block elements may not be regarded as the transition elements?
8.18 Transition elements are those elements in which the atoms or ions (in stable oxidation state) contain partially filled d-orbital. These elements in the d-block show a transition of properties between s-block and p-block. Therefore, these are called transition elements. Elements such as Zn, Cd, and Hg cannot be classified as transition elements because these have completely filled d-subshell.
8.29 Compare the stability of +2 oxidation state for the elements of the first transition series.
8.29 It can be observed from the above table that in the starting of 3d transition series elements like Sc, Ti, V, Cr in +2 state are not that stable in their elements in the +3 state.
In the middle Mn2+, Fe2+, Co2+are quite known. In fact, Mn2+ and Mn7+ are most stable states in Mn. Fe2+ is less stable when compared to Fe3+ which is due to fact that Fe3+ is able to loose one electron to acquire d5 state which has extra stability. Co2+ is less stable as compared to Co3+.
Ni2+ is the most common and stable among its +2, +3, +4 states. Cu2+ is more stable and is quite common as compared to Cu+. Towards the end, Zn forms only Zn2+ which is highly stable as it has 3d10 state.
As it becomes difficult to remove the third electron from d-orbital, the stability of +2 oxidation state increases from top to bottom.
8.23 How is the variability in oxidation states of transition metals different from that of the non transition metals? Illustrate with examples.
8.23 In transition metals, it can be observed that the oxidation state of the metal varies from +1 to +7 which is obtained by removing all its valence electrons. In transition elements the oxidation state differ by 1 unlike non transition elements whose oxidation state differs by 2, for example in transition metals (Cu2+, Cu3+ and Fe2+, Fe3+) and In non-transition elements, this variation is selective, always differing by 2, e.g. S exhibits +2, +4, +6 oxidation states and N exhibits +3, + 5, etc.
8.26 Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with (i) iron(II) ions (ii) SO2 and (iii) oxalic acid? Write the ionic equations for the reactions.
8.26 Potassium permanganate can be prepared from MnO2. It can be done by fusing the ore with KOH in presence of an oxidizing agent like atmospheric oxygen/KNO3 etc to give green coloured K2MnO4 as the product.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
The Product K2MnO4 is extracted with water and then oxidised by passing ozone/chlorine into the solution or electrolytically.
Electrolytic oxidation:
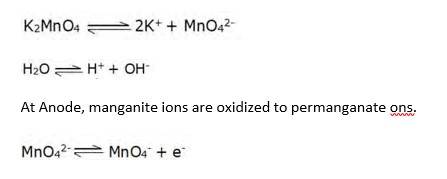
(i) Acidified KMnO4 solution oxidizes Fe(II) ions to Fe(III) ions and water as product
MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O
Fe2+→ Fe3++ e- } x5
Overall: MnO4 - + 8H+ + 5Fe2+ → Mn2+ + 4H2O + 5Fe3+
(ii) Acidified potassium permanganate oxidizes SO2 to sulphuric acid as follows
MnO4 - + 6H+ + 5e- → Mn2+ + 3H2O } x2
2H2O + 2SO2 + O2→ 4H+ + 2SO42- + 2e-} x5
Overall: MnO4 - + 4H2O + 10SO2 + 5O[2 → 8H+ + 10SO4 2- + 2H+
(iii) Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide
MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O} x2
C2O42-→ 2CO2 + 2e- } x5
Overall: 2MnO4 - + 16H++ 5C2O4 2- → 2Mn2+ + 10CO2 + 8H2O
8.46 Write down the number of 3d electrons in each of the following ions: Ti2+, V2+ , Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+. Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral).
8.46
S.no | Ion | Configuration | Number of 3d electrons | No. of unpaired Electrons | 3d orbitals |
1 | Ti2+ | 3d2 | 2 | 2 | t22g e0g |
2 | V2+ | 3d3 | 3 | 3 | t32g e0 g |
3 | Cr3+ | 3d3 | 3 | 3 | t32g e0 g |
4 | Mn2+ | 3d5 | 5 | 5 | t32g e2 g |
5 | Fe2+ | 3d6 | 6 | 4 | t42g e2 g |
6 | Fe3+ | 3d5 | 5 | 5 | t32g e2 g |
7 | Co2+ | 3d7 | 7 | 3 | t52g e2 g |
8 | Ni2+ | 3d8 | 8 | 2 | t62g e2 g |
9 | Cu2+ | 3d9 | 9 | 1 | t62g e3g
|
Note: In an octahedral field, the d-orbitals split into two sets of orbitals, the set of orbitals ( dxy, dyz, dxz) with lower energy is called t2g and the set of orbitals (dx2-y2 and dz2) with higher energy is called eg
8.19 In what way is the electronic configuration of the transition elements different from that of the non transition elements?
8.19 Transition metals have a partially filled d−orbital. Therefore, the electronic configuration of transition elements is (n−1) d1-10 ns0-2 The non-transition elements either do not have a partially filled d−orbital. Therefore, the electronic configuration of non-transition elements is ns1-2 orns2 np1-6.
8.41 Use Hund’s rule to derive the electronic configuration of Ce3+ ion, and calculate its magnetic moment on the basis of ‘spin-only’ formula.
8.41 The atomic number of Cerium (Ce) is Z = 58.
The electronic configuration of 58Ce= [Xe]54 4f1 5d1 6s2
And, the electronic configuration of Ce3+= [Xe]54 4f1 , i.e., there is only one unpaired electron, i.e., n = 1.
Now, the magnetic moment on the basis of spin only formula is given as:
μ = √n(n+2) BM
⇒ μ = √1(1+2)
⇒ μ = √3 BM
⇒ μ = 1.73 BM
Bohr magneton or BM is a unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
8.17 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
8.17 On moving along the lanthanoid series, the atomic number increases. Also, with the increase in atomic number, the number of electrons in the 4f orbital also increases.
The 4f electrons have a poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. Consequently, the force of attraction between the nucleus and valence electrons increases. This results in a decrease in the size of lanthanoids with the increase in the atomic number. This is termed as lanthanoid contraction.
CONSEQUENCES:
1. The properties of second and third transition series are similar in
2. Separation of lanthanoids from its compounds is possible due to lanthanide
3. The variation in the basic strength of lanthanide hydroxides os due to this (Basic strength decreases from La (OH)3 to Lu (OH)3)
8.47 Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
8.47 The given statement is true as explained below:
1. Atomic radii of the heavier transition elements (4d and 5d series) are larger than those of the corresponding elements of the first transition series through those of 4d and 5d series are very close to each (Lanthanoid contraction)
2. Due to stronger intermetallic bonding (M-M bonding), the melting and boiling points of heavier transition elements are greater than those of the first transition series
3. The ionization enthalpies of 5d series are higher than the corresponding elements of 3d and 4d
4. The heavier transition elements form low spin complexes whereas the elements of the first series form low spin or high spin complexes depending upon the strength of ligand field.
The strength of a ligand can be found out relating to its If a species is highly electronegative it has less tendency to donate e-s and thus is a weak field ligand and vice versa
5. +2 and +3 oxidation states are more common for elements in the first transition series, while higher oxidation states are more common for the heavier elements.
8.32 What is meant by ‘disproportionation’? Give two examples of disproportionation reaction in aqueous solution.
8.32 The reactions in which same substance gets oxidized as well as reduced due to unstable oxidation state.
Example:
2MnO42- + 4H+? 2MnO4- + MnO2 + 2H2O
Mn (VI) is oxidised to Mn (VII) and also reduced to Mn (IV).
2CrO4 3- +2H+? CrO4 2- +Cr3+ + 4H2O
Cr (V) is oxidised to Cr (VI) and also reduced to Cr (III).
8.2 In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest, i.e., 126 kJ mol–1. Why?
8.2 The enthalpy of atomization depends on the strength of the metallic bonding. Stronger the metallic bonding, greater is the enthalpy of atomization. The metallic bonding is strong when there are more unpaired electrons in the atom.
All transition metals (except Zn, electronic configuration: [Kr] 3d10 4s2), have at least one unpaired electron that is responsible for their stronger metallic bonding. Since the Zn atom does not have an unpaired electron, the metallic bonding is weak and hence the enthalpy of atomization is low.
8.33 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
8.33 Copper (29) has electronic configuration 1s22s22p63s23p63d104s1. It can easily lose one electron to give stable configuration as it has completely filled d-orbital.
8.40 Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
8.40 Last actinoid is lawrencium - 103
Electronic configuration: [Rn]86 5f14 6d1 7s2
Possible oxidation state: +3
Benefit of using NCERT Solutions for Class 12 Chemistry Chapter 4
The benefits of Class 12 Chemistry chapter 4 NCERT solutions are as follows.
- Enhance concept understanding: The solutions to the complex questions are explained in a simple way for the understanding of students. Better understanding of the question and solutions will make the concept clear.
- Improve Problem solving skills: Solving the NCERT Class 12 d and f block questions with the help of and solutions will improve the way of solving the questions.
- Revision and Practice: This is the best study material for the preparation of the exam. Solving the question will help in self-assessment and focus on the weaker topics.
NCERT Chemistry Chapter 4 The d and f Block Elements – FAQs
Students can find the frequently asked questions (faqs) on the Class 12 d and f block elements.
Explore exams which ask questions on Chemistry Ncert Solutions Class 12th
Select your preferred stream
Chemistry Ncert Solutions Class 12th Exam
