
Class 12 Chemistry Chapter 1 Solid State NCERT Solutions:
Shiksha has prepared a comprehensive solution for Class 12 Chemistry Chapter 1 Solid State from NCERT textbooks. This chapter is crucial for students to understand the arrangement of particles in solids, their properties, and the different types of solid structures. A strong understanding of the solid state is essential for learning about crystal lattices, unit cells, and the behavior of solids in various applications.
Solid is a state of matter like liquid and gas. In solid, the molecules are tightly packed and have definite mass and shape. In this chapter, students will study various properties of solids, different structures of solids, packaging structures, imperfections, and electrical & magnetic properties. Chemistry Chapter 1 Solid State covers key concepts such as Amorphous and Crystalline Solids, Unit Cells, Packing Efficiency, Imperfections in Solids, Electrical and Magnetic Properties of Solids, and Band Theory. These concepts are fundamental for understanding material science, semiconductor technology, and metallurgy. Mastering this chapter is important for CBSE Board exams and competitive exams such as JEE Main, NEET, and NDA.
Shiksha provides detailed Class 12 Chemistry Chapter 1 Solutions to help students build a strong conceptual foundation. Many questions related to crystal structures, density calculations, and types of solids frequently appear in competitive exams. We have compiled Class 12 Chemistry NCERT Solutions for Chapter 1 in PDF format, which students can easily access through the link available on this page.
Students also check the Class 12 Chapter 1 NCERT solutions, which can help to get a better understanding of several other Class 12 Chemistry chapters. Students can access the complete Class 11 Chemistry chapter-wise Solutions and Class 12 Chapter-wise Chemistry solutions on Shiksha. For more information check below;
- NCERT Chemistry Class 12 Solid State: Key Topics and Important Formulae
- NCERT Chemistry Class 12th Solution PDF - Solid State Chapter Download
- Solid State Solutions
NCERT Chemistry Class 12 Solid State: Key Topics and Important Formulae
Candidates can check here the list of all the topics that are covered in NCERT class 12 solid state chapter.
- General characteristics of solid state
- Amorphous and crystalline solids
- Classification of crystalline solids
- Molecular solids
- Ionic solids
- Metallic solids
- Covalent or network solids
- Crystal lattices and unit cells
- Primitive and centered unit cells
- Number of atoms in a unit cell
- Primitive unit cubic cells
- Body centered cubic unit cells
- Face centered cubic unit cells
- Closed package structure
- Formula of a Compound and Number of Voids Filled
- Packing efficiency
- Packing Efficiency in hcp and ccp Structures
- Efficiency of Packing in BodyCentred Cubic Structures
- Packing Efficiency in Simple Cubic Lattice
- Calculations Involving Unit Cell Dimensions
- Imperfections in Solid
- Types of Point Defects
- Electrical Properties
- Conduction of Electricity in Metals
- Conduction of Electricity in Semiconductors
- Magnetic Properties
-
Class 12 Chemistry - Solid State
The Solid State chapter is crucial for understanding the properties of solids and their classification based on structure, bonding, and defects. It is an important topic for JEE & NEET.
📌 Key Topics in Solid State
1. Types of Solids
- Crystalline Solids (Ordered arrangement, sharp melting point)
- Amorphous Solids (Disordered arrangement, gradual softening)
2. Classification of Crystalline Solids
Type of Solid Constituent Particles Binding Forces Example Ionic Ions Electrostatic NaCl, KCl Covalent (Network) Atoms Covalent Bonds Diamond, Graphite Metallic Metal ions Metallic Bonding Fe, Cu Molecular Molecules Van der Waals Forces Ice, CO₂ 3. Unit Cell and Crystal Lattice
- Unit Cell: Smallest repeating unit of a crystal lattice.
- Lattice Parameters: a, b, c (edge lengths) and α, β, γ (angles).
- Types of Unit Cells:
- Primitive (Simple Cubic)
- Body-Centered Cubic (BCC)
- Face-Centered Cubic (FCC)
- Hexagonal Close-Packed (HCP)
4. Packing Efficiency and Density
- Packing efficiency: Percentage of total volume occupied by particles.
- Density of Unit Cell: (Where Z = Number of atoms per unit cell, M = Molar mass, a = Edge length, = Avogadro's number)
5. Defects in Solids
- Point Defects:
- Schottky Defect → Equal no. of cations & anions missing (NaCl).
- Frenkel Defect → Ion displaced from normal position (AgCl).
- Impurity Defects → Foreign atoms replace original ones (e.g., doping in semiconductors).
6. Electrical and Magnetic Properties
- Conductors, Semiconductors, and Insulators: Based on band theory.
- Types of Magnetic Materials:
- Paramagnetic (Fe³⁺, O₂)
- Diamagnetic (NaCl, H₂O)
- Ferromagnetic (Fe, Co, Ni)
🔥 Important Concepts & Formulae
1. Number of Atoms in a Unit Cell
Type of Unit Cell Number of Atoms (Z) Simple Cubic (SC) 1 Body-Centered Cubic (BCC) 2 Face-Centered Cubic (FCC) 4 Hexagonal Close Packed (HCP) 6 2. Atomic Radius and Edge Length Relationships
- Simple Cubic:
- BCC:
- FCC:
3. Packing Efficiency Formulae
Lattice Type Packing Efficiency (%) Simple Cubic 52.4% BCC 68% FCC & HCP 74% 4. Density Formula
- = Density of unit cell
- = Number of atoms per unit cell
- = Molar mass
- = Edge length
- = Avogadro’s number
5. Coordination Number
Crystal Structure Coordination Number Simple Cubic 6 BCC 8 FCC & HCP 12
📌 Summary of Key Takeaways
✔ Solid State deals with structure, properties, and defects in solids.
✔ Types of solids depend on their bonding (Ionic, Covalent, Metallic, Molecular).
✔ Crystalline solids have a well-defined lattice structure.
✔ Packing efficiency, coordination number, and density are crucial for calculations.
✔ Schottky & Frenkel defects alter conductivity & density.
✔ Magnetic properties depend on electron alignment.Would you like numerical problems or concept clarifications? 😊
NCERT Chemistry Class 12th Solution PDF - Solid State Chapter Download
The Solid State chapter is an important chapter for the students because it forms a strong foundation for class XII students. It provides a glimpse of the scope of Chemistry to the students. The students can download here the Chemistry NCERT Class 12 PDF with solutions for free.
Download Here: NCERT Solution for Class XII Chemistry Solid State PDF
Solid State Solutions
| Intext Q 1.1 Why are solids rigid? |
| A 1.1 : It is because of the lack of mobility which makes a solid rigid. Since the atoms are almost lacking in mobility, their kinetic energy is negligibly small. The constituent particles in solids are held together by strong inter-atomic forces. The average location of the particles in a lattice does not change with time. |
| Intext Q 1.2 Why do solids have a definite volume? |
| A 1.2 They are rigid so the intermolecular forces of attraction that are present in solids are very strong. Hence, solids have a definite volume. |
| Intext Q 1.3 Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fiber glass, copper. |
| A 1.3 Amorphous solids: Amorphous solids are solids in which the geometry of the solid is said to be irregular. When the amorphous solids are cut with a knife, a clean surface is not obtained. The amorphous solids are called pseudo solids or sometimes also as super cooled liquids. Polyurethane, Cellophane, Polyvinyl Chloride, Fiberglass, Teflon. Crystalline solids: Crystalline solids are solids in which the geometry of the solid is said to be regular. When the Crystalline solids are cut with a knife, a clean surface is obtained. Crystalline solids are also called true solids.Naphthalene, Benzoic acid, Potassium nitrate, and Copper |
| Intext Q 1.4 Why is glass considered a super cooled liquid? |
| A 1.4 Glass is basically an amorphous solid. When glass is made the silica is cooled from its liquid state, and it does not solidifies even when the temperature is dropped below freezing point. Hence, glass is a super cooled liquid. Due to this fluidity property, glass can be considered as a liquid of extremely high viscosity. The evidence of the fact can be seen in the windows getting thicker at bottom over a period of time. |
Commonly asked questions
1.14 What is the two dimensional coordination number of a molecule in square close-packed layer?
1.14 In square close-packed layer, a molecule is in contact with four of its neighbours. Therefore, the two-dimensional coordination number of a molecule in square close-packed layer is 4.
1.30 How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain
1.30 Knowing the density of an unknown metal and the dimension of the unit cell, the atomics mass of the metal can be determined.
Let, Atomic mass of element
(M)=d* a3* NA x Z
Where, d = density
- a3= volume of the unit cell
- NA = Avogadro's number
- Z= number of atoms present in one unit cell.
- Density of the unit cell= Mass of the unit cell / Volume of the unit cell
- d= zm / a3
1.37 Niobium crystallises in body-centred cubic structure. If density is 8.55 g cm^–3, calculate atomic radius of niobium using its atomic mass 93 u.
1.37 Calculation of edge length of unit cell(a)
Atomic mass of the element (M)= 93g mol−1
Number of particles in bcc type unit cell (Z) = 2
Mass of the unit cell = Z × MNA = 2 × (93 g mol−1) (6.022×1023mol−1)
=30.89×10−23g
Density of unit cell (d) =8.55 g cm−3
Volume of unit cell (a3)=Mass of unit cell
Density of unit cell=(30.89×10−23g)(8.55 g cm−3)
=36.16×10−24cm3
Edge length of unit cell (a) = (36.13×10−24cm3)13
=3.31 × 10−8cm
Step II: Calculation of radius of unit cell (r)
For bcc structure, r=√3a4
=√3×(3.31×10−8cm)4
=1.43×10−8cm
1.42 Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p-type semiconductor?
1.42 The ratio less than 2:1 in Cu2O shows that some cuprous (Cu+) ions have been replaced by cupric (Cu+2) ions. To maintain electrical neutrality, every two Cu+ ions will be replaced by one Cu+2 ion, thereby creating a hole. As conduction will be due to the presence of these positive holes, hence it is a p -type semi conductor
1.49 If NaCl is doped with 10^–3 mol % of SrCl2, what is the concentration of cation vacancies?
1.49 NaCl is doped with 10−3 mol % of SrCl2
100 moles of NaCl are doped with 0.001 moles of SrCl2
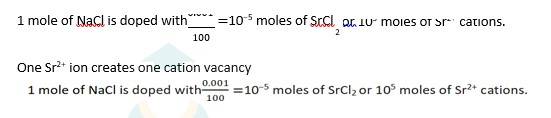
1.21 Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
1.21 Two or more cations of lower valency are replaced by a cation of higher valency to maintain electrical neutrality. Hence some cation vacancies are created. For example : In an ionic solid 'NaCl', is impurity of Sr2+ is added (as SrCl2) then two Na+ ions left thus lattice sited. To maintain electrical neutrality one lattice site is occupied by Sr2+ ion while other lattice site will remain vacant.
1.50 Explain the following with suitable examples: (i) Ferromagnetism (ii) Paramagnetism (iii) Ferrimagnetism (iv) Antiferromagnetism (v) 12-16 and 13-15 group compounds.
Ferromagnetism: The substances that are strongly attracted by a magnetic field are called ferromagnetic substances can be permanently magnetised even in the absence of a magnetic field. Some examples of ferromagnetic substances are iron, cobalt, nickel, gadolinium, and CrO2. In solid state, the metal ions of ferromagnetic substances are grouped together into small regions called domains and each domain acts as a tiny magnet. In an un-magnetised piece of a ferromagnetic substance, the domains are randomly-oriented and so, their magnetic moments get cancelled. However, when the substance is placed in a magnetic field, all the domains get oriented in the direction of the magnetic field. As a result, a strong magnetic effect is produced. This ordering of domains persists even after the removal of the magnetic field. Thus, the ferromagnetic substance becomes a permanent magnet.
Paramagnetism: The substances that are attracted by a magnetic field are called paramagnetic substances. Some examples of paramagnetic substances are O2, Cu2t, Fe3t, and Cr3t. Paramagnetic substances get magnetised in a magnetic field in the same direction, but lose magnetism when the magnetic field is removed. To undergo paramagnetism, a substance must have one or more unpaired electrons. This is because the unpaired electrons are attracted by a magnetic field, thereby causing paramagnetism.
Ferrimagnetism: The substances in which the magnetic moments of the domains are aligned in parallel and anti-parallel directions, in unequal numbers, are said to have Examples include Fe3O4 (magnetite), ferrites such as MgFe2O4 and ZnFe2O4. Ferrimagnetic substances are weakly attracted by a magnetic field as compared to ferromagnetic substances. On heating, these substances become paramagnetic.
Antiferromagnetism: Antiferromagnetic substances have domain structures similar to ferromagnetic substances, but are oppositely-oriented. The oppositely-oriented domains cancel out each other's magnetic
12-16 and 13-15 group compounds: The 12-16 group compounds are prepared by combining group 12 and group 16 elements and the 13-15 group compounds are prepared by combining group 13 and group15 elements. These compounds are prepared to stimulate average valence of four as in Ge or Si. Indium (III) antimonide (IrSb), aluminum phosphide (AlP), and gallium arsenide (GaAS) are typical compounds of groups 13-15. GaAs semiconductors have a very fast response time and have revolutionised the designing of semiconductor devices. Examples of group 12-16 compounds include zinc sulphide (ZnS), cadmium sulphide (CdS), cadmium selenide (CdSe), and mercury (II) telluride (HgTe). The bonds in these compounds are not perfectly covalent. The ionic character of the bonds depends on the electronegativities of the two elements.
1.18 An element with molar mass 2.7×10-2 kg mol-1 forms a cubic unit cell with edge length 405 pm. If its density is 2.7×103 kg m-3, what is the nature of the cubic unit cell?
1.18 Molar mass of the element = 2.7*10-2 kg mol-1
Edge length, a = 405 pm
Density, d = 2.7*103 kg m-3
Using the formula, d=? *? / ? 3NA
Putting the values given at their appropriate place, we get
(2.7 X 103 )X (405 X 10-12)3 X 6.022 X 10 / 2.7*10-2 = 3.99 which is approximately equal to 4
Therefore, it is an fcc unit cell
1.38 If the radius of the octahedral void is r and radius of the atoms in close packing is R, derive relation between r and R.
1.38 We apply pythagoras theorem AC2= AB2+ BC2
(2R)2= (R+r)2 +(R+r)2 = 2(R+r)2
4R2 = 2(R+r)2
(2R)2= (R+r)2
√2(R)2= √(R+r)2 = √2r = R+r
r = √2 R – R
r = (√2-1) R
r = (1.4114-1)R
r= 0.414 R
1.3 Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fiber glass, copper.
1.3 Amorphous solids: Amorphous solids are solids in which the geometry of the solid is said to be irregular. When the amorphous solids are cut with a knife, a clean surface is not obtained. The amorphous solids are called pseudo solids or sometimes also as super cooled liquids. Polyurethane, Cellophane, Polyvinyl Chloride, Fiberglass, Teflon.
Crystalline solids: Crystalline solids are solids in which the geometry of the solid is said to be regular. When the Crystalline solids are cut with a knife, a clean surface is obtained. Crystalline solids are also called true solids.Naphthalene, Benzoic acid, Potassium nitrate, and Copper
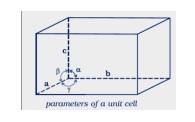
1.11 Name the parameters that characterize a unit cell.
1.11 The six parameters that characterize a unit cell are as follows.
(i) Its dimensions along the three edges, a, b, and c these edges may or may not be equal.
(ii) Angles between the edges these are the angles (between edges b and c), (between edges a and c), and (between edges a and b)
1.16 A compound is formed by two elements M and N. The element N forms ccp and atoms of M occupy 1/3rd of tetrahedral voids. What is the formula of the compound?
1.16 The atoms of element M occupy 1/3rd of the tetrahedral voids.
Therefore, the number of atoms of M is equal to 2 1/3 = 2/3rd of the number of atoms of N. Therefore, ratio of the number of atoms of M to that of N is M : N = (2/3):1 = 2:3 Thus, the formula of the compound is M2N3.
ccp= fcc = 6 * 1/2 + 8 X 1/8 = 4 = N atoms
Tetrahedral void= 8 (in fcc)
Number of M atoms = 8/3
So empirical formula = M 8/3 N2 = M2N3
1.1 Why are solids rigid?
1.1 : It is because of the lack of mobility which makes a solid rigid. Since the atoms are almost lacking in mobility, their kinetic energy is negligibly small. The constituent particles in solids are held together by strong inter-atomic forces. The average location of the particles in a lattice does not change with time.
1.2 Why do solids have a definite volume?
1.2 They are rigid so the intermolecular forces of attraction that are present in solids are very strong. Hence, solids have a definite volume.
1.4 Why is glass considered a super cooled liquid?
1.4 Glass is basically an amorphous solid. When glass is made the silica is cooled from its liquid state, and it does not solidifies even when the temperature is dropped below freezing point. Hence, glass is a super cooled liquid. Due to this fluidity property, glass can be considered as a liquid of extremely high viscosity. The evidence of the fact can be seen in the windows getting thicker at bottom over a period of time.
1.5 Refractive index of a solid is observed to have the same value along all directions.Comment on the nature of this solid. Would it show cleavage property?
1.5 As isotropic solid has the same value of physical properties when measured along different directions. Therefore, the given solid, having the same value of refractive index along all directions, is isotropic in nature. Hence, the solid is and amorphous solid. When an amorphous solid is cut with a sharp edged tool, it cuts into two pieces with irregular surfaces
1.6 Classify the following solids in different categories based on the nature of Intermolecular forces operating in them:
1.6 Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite, rubidium, argon, silicon carbide. Solids have been classified into different categories based on chemical bonding. The chemical bonding depends on the intermolecular forces of attraction between the atoms.
a) Potassium sulphate →Ionic solid
b) Tin→ Metallic solid
c) Benzene→ Molecular (non-polar) solid
d) Urea→ Polar molecular solid
e) Ammonia→ Solid ammonia is a hydrogen-bonded molecular solid which is also known as polar molecular solid
f) Water→ Hydrogen bonded molecular solid
g) Zinc sulphide→ Ionic solid
h) Graphite→ Covalent or network solid
i) Rubidium→ Metallic solid
j) Argon→ Non-polar molecular solid
k) Silicon carbide →Covalent or network solid
1.7 Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
1.7 The given properties are the resource of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solid are quartz (SiO) and diamond (C).
1.8 given properties are the resource of a covalent or network solid. Therefore, the given solid is a covalent or network solid. Examples of such solid are quartz (SiO) and diamond(C).
1.8 In solid state, ions are held together by strong electrostatic forces and are not free to move about within the solid. In ionic compounds, electricity is conducted by ions. Hence, in molten state or in solution form, the ions are free to move and can conduct electricity
1.9 What type of solids are electrical conductors, malleable and ductile?
1.9 Metallic solids are electrical conductors, malleable, and ductile.
1.10 Give the significance of a ‘lattice point’
1.10 The significance of a lattice point is that each lattice point represents one constituent particle of a solid which may be an atom, a molecule (group of atom), or an ion.
1.12 Distinguish between (i) Hexagonal and monoclinic unit cells (ii) Face-centered and end-centered unit cells.
1.12

(ii) Face-centered unit cell: In a face-centered unit cell, the constituent particles are present at the corners
and one at the centre of each face.
End-centered unit cell: An end-centered unit cell contains particles at the corners and one at the centre of
any two opposite faces.
1.13 Explain how much portion of an atom located at (i) corner and (ii) body centre of a cubic unit cell is part of its neighbouring unit cell.
1.13 (i) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore, 1/8th portion of the atom is shared by one unit cell.
(ii) An atom located at the body centre of a cubic unit cell is not shared by its neighbouring unit cell. Therefore, the atom belongs only to the unit cell in which it is present i.e., its contribution to the unit cell is 1
1.15 A compound forms hexagonal close-packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
1.15 Number of atoms in close packaging = 0.5 mol
1 atom has 6.022*1023 particles
So Number of particles in close-packed = 0.5 * 6.022 * 1023 = 3.011*1023
Number of tetrahedral voids = 2 * number of atoms in close packaging
Number of tetrahedral voids = 2 * 3.011 * 1023= 6.022*1023
Number of octahedral voids = number of atoms in close packaging
So the number of octahedral voids = 3.011 * 1023
Total number of voids = Tetrahedral void + octahedral void
=6.022 * 1023 + 3.011 * 1023= 9.03*1023
1.17 Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body-centered cubic and (iii) hexagonal close-packed lattice?
1.17 Hexagonal close-packed lattice has the highest packing efficiency of 74%. The packing efficiencies of simple cubic and body-centered cubic lattices are 52.4% and 68% respectively
1.19 What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
1.19 When a solid is heated, vacancy defect can arise. A solid crystal is said to have vacancy defect when some of the lattice sites are vacant. Vacancydefect leads to a decrease in the density of the solid.
1.20 What type of stoichiometric defect is shown by: (i) ZnS (ii) AgBr
1.20 ZnS shows Frenkel Defect.
AgBr shows Frenkel Defect and Schottky Defect. Frenkel Defect: It is a kind of defect in crystalline solids in which atoms are displaced from their lattice position
to interstitial site creating vacancy at the lattice point. It usually occurs in ionic solid with large difference in size
of ions.
Schottky Defect: This defect occurs when oppositely charged ions leave their lattice site creating vacancies in
such a way that electrical neutrality of crystal is maintained. It is generally seen in highly ionic compounds where
difference in size of cation and anion is small.
1.22 Ionic solids, which have anionic vacancies due to metal excess defect, develop colour. Explain with the help of a suitable example
1.22 The colour develops because of the presence of electrons in the anionic sites. These electrons absorb energy from the visible part of radiation and get excited. For example, when crystals of NaCl are heated in an atmosphere of sodium vapours, the sodium atoms get deposited on the surface of the crystal and the chloride ions from the crystal diffuse to the surface to form NaCl with the deposited Na atoms. During this process, the Na atoms on the surface lose electrons to form Na+ ions and the released electrons diffuse into the crystal to occupy the vacant anionic sites. These electrons get excited by absorbing energy from the visible light and impart yellow colour to the crystals
1.23 A group 14 element is to be converted into n-type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
1.23 An n-type semiconductor conducts because of the presence of extra electrons. Therefore, a group 14 element can be converted to n-type semiconductor by doping it with a group 15 element.
1.24 What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your answer.
1.24 Ferromagnetic substances would make better permanent magnets because when the ferromagnetic substance is placed in a magnetic field, all domains get oriented in the direction of magnetic field and strong a magnetic effect is produced.
1.25 Define the term 'amorphous'. Give a few examples of amorphous solids
1.25 Amorphous solids have short-range order with irregular shapes of constituent particles. They have isotropic nature and melt over a range of temperatures. They do not have a definite enthalpy of fusion. Examples of amorphous solids are glass, rubber, plastic, etc.
1.26 What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
1.26 Quartzis crystalline solid with long range order and glass is amorphous solid (or pseudo solid or super cooled liquid) with short range order and has a tendency to flow. When quartzis heated, it can be converted into glass.
1.27 Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous. (i) Tetra phosphorus decoxide (P4O10) (ii) Ammonium phosphate (NH4) 3PO4 (iii) SiC (iv) I2 (v) P4 (vi) Plastic (vii) Graphite (viii) Brass (ix) Rb (x) LiBr (xi) Si
1.27 The different solids are classified below:
a) Ionic solids: Ammonium phosphate (NH4)3PO4), LiBr
b) Metallic solid: Brass, Rb
c) Molecular solids: Tetraphosphorous decaoxide (P4O10), Iodine (I2), P4
d) Network (covalent) solids: Graphite, SiC, Si
e) Amorphous solid: Plastics
1.28 (i) What is meant by the term 'coordination number'? (ii) What is the coordination number of atoms: (a) in a cubic close-packed structure? (b) in a body-centred cubic structure?
1.28 Coordination number is the number of the nearest neighbors with which a given atom is in contact. In an
ionic crystal, the coordination number of an ion refers to the number of oppositely charged ions that
surround that ion.
The coordination number of atoms in a
(a) Cubic close-packed structure is 12.
(b) Body-centred cubic structure is 8
1.30 'Stability of a crystal is reflected in the magnitude of its melting points'. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
1.30 Stability of a crystal is directly proportional to the magnitude of its melting points. Higher is the magnitude of forces holding the constituent particles together, higher will be the melting point and higher will be the stability. Thus ionic crystals (NaCl, KNO3 etc.) have very high melting points and stable crystal lattices. On other hand, molecular solids (naphthalene, iodine etc.) have low melting points and low stability.
The melting points of solid water, ethyl alcohol, diethyl ether and methane are 273 K, 155.8 K, 156.8 K and 90.5 K respectively.Solid water and ethyl alcohol have higher melting points due to presence of inter-molecular hydrogen bonds. Since the extent of hydrogen bonding in solid water is greater than the extent of hydrogen bonding in ethyl alcohol, the melting point of solid water is higher than the melting point of ethyl alcohol.
Polar diethyl ether involves dipole-dipole interactions. Non polar methane involves weak van there Waals forces (London dispersion forces) which are weaker than dipole-dipole interactions. Hence, the melting point of methane is much lower than the melting point of diethyl ether.
1.31 How will you distinguish between the following pairs of terms: (i) Hexagonal close-packing and cubic close-packing? (ii) Crystal lattice and unit cell? (iii) Tetrahedral void and octahedral void?
1.31 (i) In hexagonal close packing (hcp), the spheres of the third layer are vertically above the spheres of the first layer. It means tetrahedral voids of the second layer are covered by the spheres of the third layer. The AB. type.In cubic close packing (ccp), the spheres of third layer cover the octahedral voids of second layer. But the spheres of the fourth layers are aligned with those of the first layer. The pattern is ABC. type.
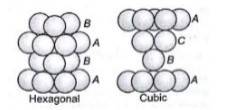
(ii) Crystal lattice is the three dimensional arrangement of identical point in the space which represent how the constituent particles (atoms, ions, molecules) are arranged in a crystal.Unit cell is the smallest portion of a crystal lattice which, when repeated in different directions, generates the entire lattice
(iii) Tetrahedral voids are surrounded by four spheres which lie at the vertices of a regular tetrahedron. There are 2 tetrahedral voids per atom in a crystal.
Octahedral voids are surrounded by six spheres and formed by a combination of two triangular voids of the first and second layer. There is one octahedral void per atom in a crystal.
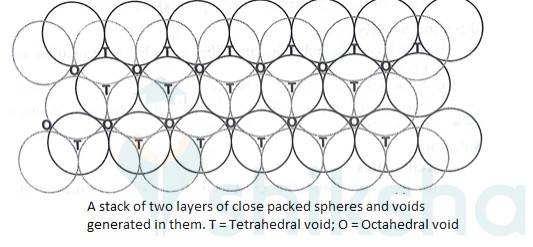
1.32 How many lattice points are there in one unit cell of each of the following lattice? (i) Face-centred cubic (ii) Face-centred tetragonal (iii) Body-centred
1.32 Number of corner atoms per unit cell
= 8 corners atoms ×18 atom per unit cell
=8 × 18 = 1 atom
Number of face face centred atoms per unit cell
= 6 face centred atoms × 12 atom per unit cell
= 6 × 12 = 3 atoms
∴ Total number of atoms (lattice point) = 1 + 3 = 4
(ii) As in (i)
No. of lattice points = 4
(iii) In bcc unit cell, number of corner atoms per unit cell
=8 corners×18 per corner atom
=8×18=1 atom
Number of atoms at body centre =1×1 = 1 atom
∴ Total number of atoms (lattice points) = 1 + 1 =
1.33 Explain (i) The basis of similarities and differences between metallic and ionic crystals. (ii) Ionic solids are hard and brittle.
(i) Both metallic and ionic crystals have strong forces of attraction between their atoms or ions. In metallic crystals there is a strong metallic bond present between electrons and positively charged ions. In ionic crystals there are strong ionic bonds between anions and cations. Both of them conduct electricity,
but metallic crystals conduct electricity in all three states of matter while ionic crystals conduct electricity only in molten state.
(ii) The ionic solids are hard and brittle because they have strong electrostatic forces of attraction between the anions and cations .As a result the anions and cations are tightly held by these forces and due to which they are unable to move and they are fixed at one position. So when force is applied to break them, they appear to be hard
1.34 Calculate the efficiency of packing in case of a metal crystal for (i) Simple cubic (ii) Body-centred cubic (iii) Face-centred cubic (with the assumptions that atoms are touching each other).
1.34 (i) The efficiency of packing in case of simple cubic unit cell is given below:
A simple cubic unit cell contains one atom per unit cell.
Also, a=2r, where a is the edge length and r is the radius of atom. Total volume of unit cell = a3
Packing efficiency = Volume of one sphere / Total volume of unit cell × 100
Packing Effieciency = 4/3πr3 / 8r3 X 100 = 52.4%
(ii) The efficiency of packing in case of body-centred cubic unit cell is given below:
A body-centred cubic unit cell containstwo atoms per unit cell.
Also, √3a = 4r,where a isthe edge length and r isthe radius of atom.
Total volume of unit cell = a3
Packing efficiency = Volume of one sphere / Total volume of unit cell × 100
Packing efficiency = 16/3πr3 / 16√2r3 x 100 = 74%
1.35 Silver crystallises in fcc lattice. If edge length of the cell is 4.07 × 10^–8 cm and density is 10.5 g cm^-3, calculate the atomic mass of silver.
1.35 P= density
A= edge length of the cell
NA= Avogadro Number
Z= no. of atoms in F.C.C unit cell
M= mass of the metal
Edge length of the cell = d = 4.07*10-8 cm
Density = P =10.5g/cm3
No. of unit cell of face centered cubic (F.C.C) lattice is 4, Z=4
Avogadro Number (NA) = 6.022*1023
Mass of silver = M=?

1.36 A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-centre. What is the formula of the compound? What are the coordination numbers of P and Q?
1.36 It is given that the atoms of Q are present at the corners of the cube.
Therefore, number of atoms of Q in one unit cell = 8 x 1/8 = 1
It is also given that the atoms of P are present at the body-centre.
Therefore, number of atoms of P in one unit cell = 1
This means that the ratio of the number of P atoms to the number of Q atoms, P:Q = 1:1
Hence, the formula of the compound is PQ.
The coordination number of both P and Q is 8.
1.40 Please find question below
Analysis shows that nickel oxide has the formula Ni0.98 O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
1.40 It is given that nickel oxide has the formula as Ni0.98 O1.00.
As per the formula, there are 98 Ni ions for 100 oxide ions.
Out of 98 Ni ions, let x ions be in +2 oxidation state
98−x ions will be in +3 oxidation state.
Oxide ion has −2 charge.
To maintain electrical neutrality, total positive charge on cations = total negative charge on anions.
2x+3(98−x)+100(−2)=0
x=94
Fraction of Ni2+ ions = 94/98 = 0.96
Fraction of Ni2+ ions = 98-94/98 = 0.04
Hence, the fractions of nickel that exists as Ni2+ and Ni3+ are 0.96 and 0.04 respectively
1.41 What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
1.41 These solids have conductive in the intermediate range from 10−6 to 104ohm−1m−1. As there is rise in
the temperature, conductivity also increases because electrons from the valence band jump to
conduction band.
Types of semiconductors
(a) n - type semiconductor when silicon or germanium crystal is doped with group 15 element like P or
As, the dopant atom forms four covalent bonds like a Si or Ge atom but the fifth electron no used in
bonding, becomes delocalised and contribute its share towards electrical conduction. Thus, silicon or
germanium doped with P or As is called n-type semiconductor (negative - type).
(b) p - type semiconductor When silicon or germanium is doped with group 13 element like B or Al. The
dopant atom forms three covalent bond like a B or Al atom, but at the place of fourth electron a hole is
created. Here, this hole moves through the crystal like a positive charge giving rise to electrical
conductivity.
Thus, Si or Ge doped with B or Al is called p-type of semiconductor, (p stands for positive hole) since, it is
the positive hole that is responsible for conduction
1.43 Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
1.43 There is one octahedral hole for each atom in hexagonal close packed arrangement. If the number of oxide ions (O2−) per unit cell is 1, then the number of

1.44 Classify each of the following as being either a p-type or a n-type semiconductor: (i) Ge doped with In (ii) Si doped with B
1.44 Ge is group 14 element and In is group 13 element. Hence an electron deficient hole is created and therefore, it is p–type.
2. B is group 13 elements and Si is group 14 elements, there will be a free electron. Hence, it is n-type
1.45 Gold (atomic radius = 0.144 nm) crystallises in a face-centred unit cell. What is the length of a side of the cell?
1.45 For fcc unit cell, a=2√2?.
Here, a is the edge length and r is the atomic radius (0.144 nm).
a = 2√2 ×0.144 = 0.407 nm
Hence, the length of a side of a cell is 0.407 nm.
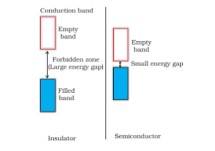
1.47 In terms of band theory, what is the difference (i) Between a conductor and an insulator (ii) Between a conductor and a semiconductor?
1.47 The energy gap between the valence band and conduction band in an insulator is very large while in a conductor, the energy gap is very small or there is overlapping between valence band and conduction band.
(ii) In a conductor, the valence band is practically filled or there is overlapping between valence band and conduction band while in semiconductor, there is always a small energy gap between them.
1.47 Explain the following terms with suitable examples: (i) Schottky defect (ii) Frenkel defect (iii) Interstitials and (iv) F-centres
Schottky defect: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. It decreases the density of a substance. Significant number of Schottky defects is present in ionic solids. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. Ionic substances containing similar-sized cations and anions show this type of defect. For example: NaCl, KCl, CsCl, AgBr, etc.
Frenkel defect: Ionic solids containing large differences in the sizes of ions show this type of defect. When the smaller ion (usually cation) is dislocated from its normal site to an interstitial site, Frenkel defect is created. It creates a vacancy defect as well as an interstitial defect. Frenkel defect is also known as dislocation defect. Ionic solids such as AgCl, AgBr, AgI, and ZnS show this type of defect.
Interstitials: Interstitial defect is shown by non-ionic solids. This type of defect is created when some constituent particles (atoms or molecules) occupy an interstitial site of the crystal. The density of a substance increases because of this defect.
F-centres: When the anionic sites of a crystal are occupied by unpaired electrons, the ionic sites are called F-centres. These unpaired electrons impart colour to the crystals. For example, when crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms are deposited on the surface of the crystal. The Cl ions diffuse from the crystal to its surface and combine with Na atoms, forming NaCl. During this process, the Na atoms on the surface of the crystal lose electrons. These released electrons diffuse into the crystal and occupy the vacant anionic sites, creating F-centres.
1.48 Aluminium crystallises in a cubic close-packed structure. Its metallic radius is 125 pm. (i) What is the length of the side of the unit cell? (ii) How many unit cells are there in 1.00 cm^3 of aluminium?
1.48 It is given that aluminum crystallises in a cubic closed packed structure.
Its metallic radius is 125 pm.
For cubic close-packed structure
a=2√2r=2√2×125=354 pm
Here, a is the edge length of the unit cell and r is the atomic radius.
(ii) Volume of one unit cell = a3 =(354 pm)3=4.4×10−23cm3(1 pm=10−10cm)
Number of unit cells in 1.00cm3= 1.00 cm3 / 4.4×10-23 cm3
= 2.27×1022
1.38 Copper crystallises into a fcc lattice with edge length 3.61 × 10^–8 cm. Show that the calculated density is in agreement with its measured value of 8.92 g cm^–3.
1.38 Kindly consider the following

Explore exams which ask questions on Chemistry Ncert Solutions Class 12th
Select your preferred stream
Chemistry Ncert Solutions Class 12th Exam
