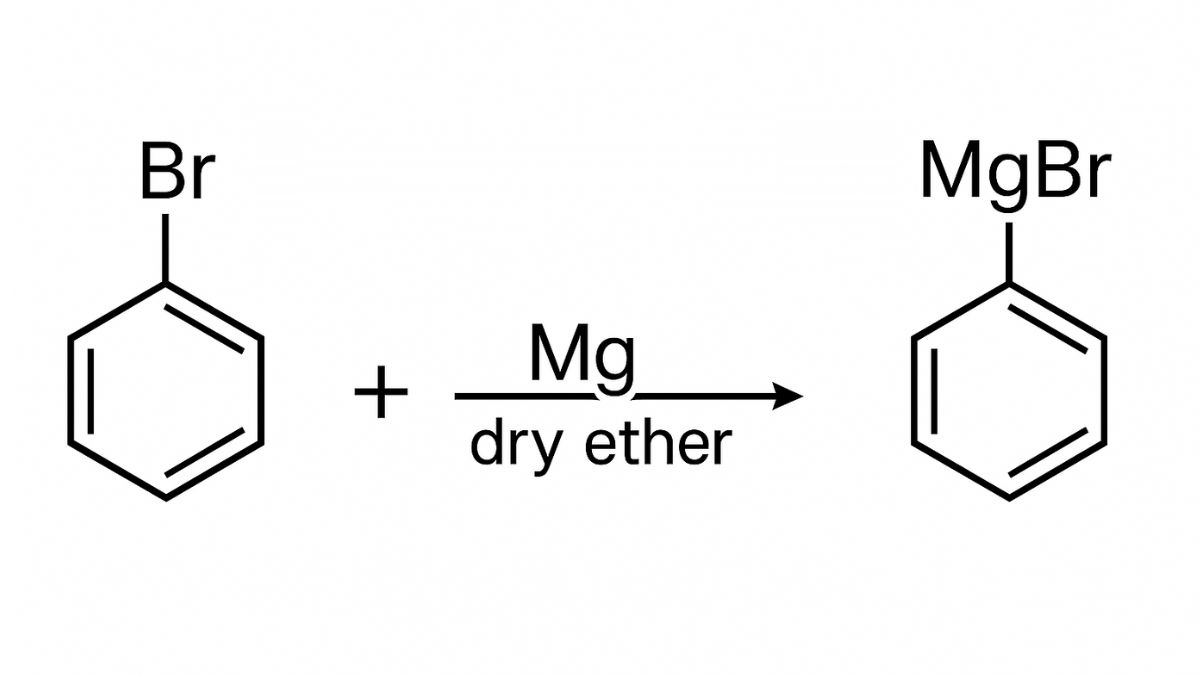
Haloalkanes and Haloarenes NCERT Solutions include the study of important methods of preparation, chemical and physical properties, and uses of organohalogen compounds. The chapter states that in an aliphatic or aromatic hydrocarbon, the replacement of a hydrogen atom(s) by halogen atom(s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
After preparing from the haloalkanes and haloarenes class 12 NCERT Solutions, students will be able to describe the reactions involved in the preparation of haloalkanes and haloarenes, name the haloalkanes and haloarenes according to the IUPAC system of nomenclature, appreciate the applications of organo-metallic compounds, and understand the environmental effects of polyhalogen compounds. Students can rely on the NCERT solutions provided by Shiksha for their exam preparation.
- NCERT Class 12 Haloalkanes and Haloarenes: Key Topics, Weightage and Important Reactions
- Haloalkanes and Haloarenes Class 12 NCERT Solutions – Free PDF Download
- Class 12 Chemistry Haloalkanes and Haloarenes NCERT Solutions
NCERT Class 12 Haloalkanes and Haloarenes: Key Topics, Weightage and Important Reactions
When preparing the Class 12 Chemistry Haloalkanes and Haloarenes NCERT solutions, students must understand the haloalkanes and haloarenes reactions, such as elimination, nucleophilic substitution, and reactions with metals. These are the topics covered in this chapter:
| Exercise | Topics Covered |
|---|---|
| 6.1 | Classification |
| 6.2 | Nomenclature |
| 6.3 | Nature of C-X Bond |
| 6.4 | Methods of Preparation of Haloalkanes |
| 6.5 | Preparation of Haloarenes |
| 6.6 | Physical Properties |
| 6.7 | Chemical Reactions |
| 6.8 | Polyhalogen Compounds |
Haloalkanes and Haloarenes Class 12 Weightage in NEET, JEE Main Exams
| Exam | Number of Questions |
|---|---|
| NEET | 1-3 questions |
| JEE Main | 1-2 questions |
Important Reactions of Haloalkane and Haloarene for CBSE and Competitive Exams
Haloalkanes and haloarenes undergo several important reactions, including nucleophilic substitution, elimination, and reactions involving metals. Students can check the key reactions below;
Reactions of Haloalkanes
- Nucleophilic Substitution Reactions: As a result, halogen exchange places with hydroxy (OH) to make alchohols.
- Elimination Reactions (E1 and E2): (Dehydrohalogenation leading to alkene formation)
- Reaction with Metals
- Wurtz Reaction: Formation of higher alkanes while reacting with Metals
-
- Grignard Reagent Formation
-
- Reduction to Alkanes: Using Zn/HCl or hydrogenation with Pd-C catalyst:
Reactions of Haloarenes
- Nucleophilic Substitution Reaction (Unusual due to resonance stabilization)
Activated in the presence of electron-withdrawing groups (e.g., -NO₂).
- Electrophilic Substitution Reactions
- Halogens direct incoming groups to ortho-para positions due to +M effect.
- Wurtz-Fittig Reaction (Haloarene + Haloalkane + Na → Alkylated Benzene)
- Fittig Reaction (Coupling of haloarenes)
Haloalkanes and Haloarenes Class 12 NCERT Solutions – Free PDF Download
Students can download the Haloalkanes and Haloarenes NCERT PDF from here to improve their exam preparation and score well in the CBSE Board exam and competitive exams like NEET and JEE Mains.
NCERT Solution Class 12 Chemistry Haloalkanes and Haloarenes PDF: Download Free PDF
More Links
| Class 12 Chemistry NCERT Solutions | NCERT Class 12 Notes | NCERT Class 12 Chemistry Notes for CBSE |
Class 12 Chemistry Haloalkanes and Haloarenes NCERT Solutions
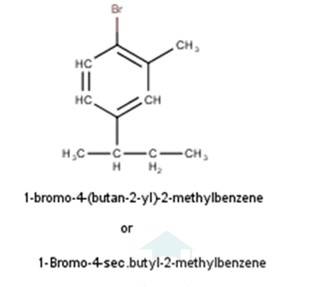
| Intext Q 10.1 Write structures of the following compounds: (i) 2-Chloro-3-methylpentane (ii) 1-Chloro-4- ethylcyclohexane (iii) 4-tert. Butyl-3-iodoheptane (iv) 1,4-Dibromobut-2-ene (v) 1-Bromo-4-sec. butyl- 2-methylbenzene. |
| A 10.1 From the name of the compound it is clear that the parent ring is pentane and chloro and methyl groups are attached in the straight chain at 2nd and 5th position respectively. Hence, the structure is as follows: From the name of the compound given it is clear that the parent group is hexane with 2 attachments namely chloro and ethyl groups at 1 and 4 positions respectively. Hence, the structure is as follows: - From the name of the compound given it is clear that the parent group is heptane with tertiary butyl and iodine groups attached at 4 and 3 positions respectively. Hence, the structure is as follows: - From the name of the compound given it is clear that the parent group is an alkene with the unsaturation present in between 2nd and 3rd carbon and 2 bromine atoms attached at 1st and 4th carbon. Hence, the structure is as follows : - From the name of the compound given it is clear that the parent group is benzene with bromine group attached at 1st carbon, secondary butyl group attached at 4th carbon and a methyl group attached at 2nd carbon. Hence, the structure is a follows : - |
| Intext Q 10.2 Why is sulphuric acid not used during the reaction of alcohols with KI? |
| A 10.2 In the presence of sulphuric acid (H2SO4), KI produces HI as follows : - 2KI + H2SO4 → 2KHSO4 + 2KI Since H2SO4 is an oxidizing agent, it oxidizes HI (produced in the reaction to I2) as follows: - 2HI + H2SO4 → I2 + SO2 +; As a result, the reaction between an alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidizing acid such as H3PO4 is used in the reaction to get the desired product. |
| Intext Q 10.3 Write structures of different dihalogen derivatives of propane. |
| A 10.3 There are four different dihalogen derivatives of propane. The structures of these derivatives are as shown below: - |
| Intext Q 10.4 Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields (i) A single monochloride. (ii) Three isomeric monochlorides. (iii) Four isomeric monochlorides |
| A 10.4 To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because of the fact that replacement of any H-atom leads to the formation of the same product. Therefore the isomer is 2,2-dimethylpropane. To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms. Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane as shown below : - To have four isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain four different types of H-atoms in it. Therefore, the required isomer is 2-methylbutane. It can be observed that there are four different types of H-atoms labelled as a, b, c, and d in 2-methylbutane as shown below: - |
Commonly asked questions
10.9 Identify A, B, C, D, E, R and R1 in the following:
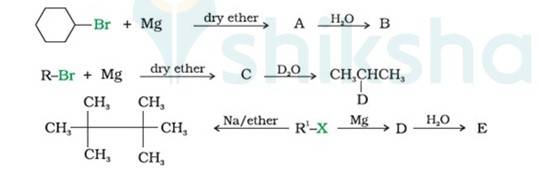
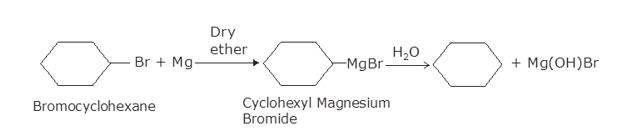
(i) Bromocyclohexane is reacting with the metal Mg to give organo-metallic compound/Grignard reagent
i.e. Cyclohexyl Magnesium Bromide (A).
Grignard reagents are highly reactive with hydrocarbons to give alkanes. Cyclohexyl Magnesium Bromide reacts with water to cyclohexane (B) and Mg (OH)Br.
(ii) As the above reaction, haloalkane with metal Mg react to form the Grignard reagent, alkyl magnesium halide, RMgX, and further with hydrocarbon gives alkane.
∴ C is CH3CH (MgBr)CH3, a Grignard Reagent, R is CH3CH (Br)CH3 and D ≅ H
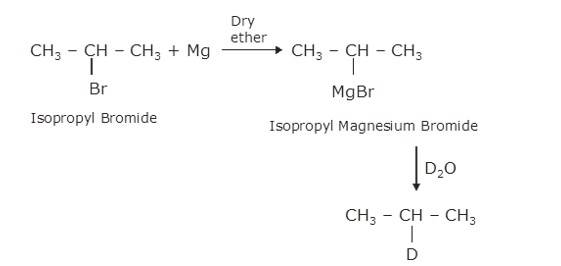
(iii) In the Wurtz Reaction, alkyl halide in the presence of sodium in dry ether gives the double number of
Now, in the reaction 2,2,3,3-tetramethyl butane is formed after the alkyl halide reacting in the presence of sodium in dry ether.
∴ Number of carbons will be 3 in R1 –X is CH3C (X) (CH3)CH3
And the remaining reaction is same as the above i.e. alkyl halide reacting with Magnesium to give Grignard Reagent (D) and further with hydrocarbon H2O to give alkane (E)
∴ D is CH3C (CH3) (CH3)MgBr E is CH3CH (CH3)CH3

10.18 Which compound in each of the following pairs will react faster in SN2 reaction with –OH?
(i) CH3Br or CH3 I (ii) (CH3 ) 3CCl or CH3Cl
(i) Since I- ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH- ion.
Better the leaving group, faster is the SN2 reaction.
(ii) On steric grounds, 1? alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3Cl will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH- ion. (Bulkier the group, slower is the SN2 )
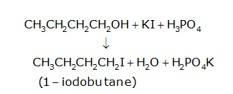
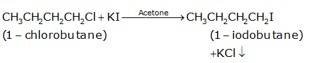
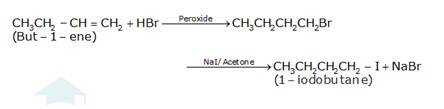
10.16 Write the equations for the preparation of 1-iodobutane from (i) 1-Butanol (ii) 1-Chlorobutane (iii) But-1-ene.
(a) 1-butanol is treated with KI in the presence of H3PO4 where the OH is being replaced by the iodine and gives H2O and KH2PO4 as the by-product with 1-iodobutane as the final
(b) The conversion of 1-chlorobutane to 1-iodobutane simply takes place by treating the reactant with KI in the presence of acetone. The iodine from KI normally replaces the chlorine from the reactant and gives 1-iodobutane as the final product with KCl as the by product.
(c) The conversion of but-1-ene to 1-iodobutane takes place according to anti-markovnikoff (positive charged ion H+ goes to the carbon which has less number of hydrogens and negative part Br- goes to the carbon which has more number of hydrogens.) i.e. the attack of HBr on but-1-ene in the presence of peroxide gives 1-bromobutane as the intermediate which on treatment with NaI+Acetone gives 1- iodobutane as the final product and NaBr as the by-product.
10.26 Arrange the compounds of each set in order of reactivity towards S N2 displacement: (i) 2- Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane (ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane (iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1- Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
The reaction involves the approaching of the nucleophile to the carbon atom to which the leaving group is attached. When the nucleophile is sterically hindered (does not have any free space or very crowded), then the reactivity towards SN2 displacement decreases. Due to the presence of substituents, hindrance to the approaching nucleophile increases in the following order.
- Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane. The structures are shown below:
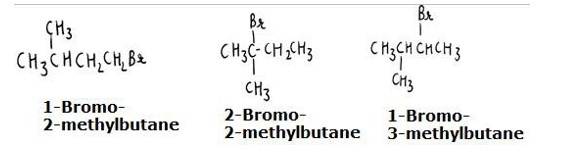
Hence, the increasing order of reactivity towards SN2 displacement is:
- Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
- The stearic hinderance in alkyl halides increases in the order of 1° < 2° < 3°, the increasing order of reactivity towards SN2 displacement is given as-
3° < 2° < 1°.
The structures are given below:

Hence, the given set of compounds can be arranged in the increasing order of their reactivity towards SN2 displacement as:
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
- The stearic hinderance to the nucleophile in the SN2 mechanism increases with a decrease in the distance of the substituents from the atom containing the leaving group. Further, the stearic hinderance increases with an increase in the number of Therefore, the increasing order of steric hindrances in the given compounds is as below:

Hence, the increasing order of reactivity of the given compounds towards SN2 displacement
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1- Bromobutane
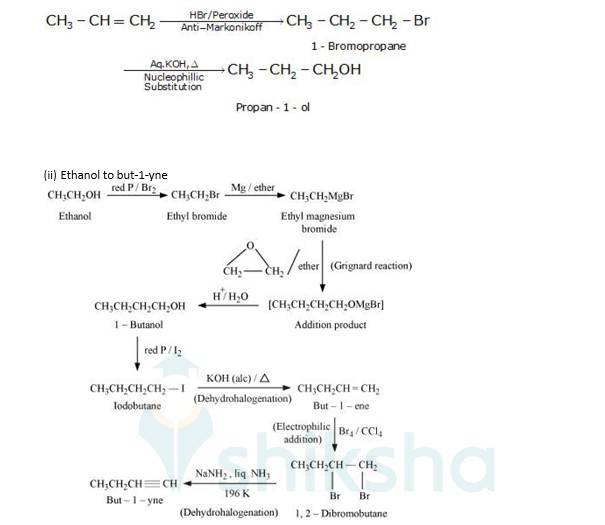
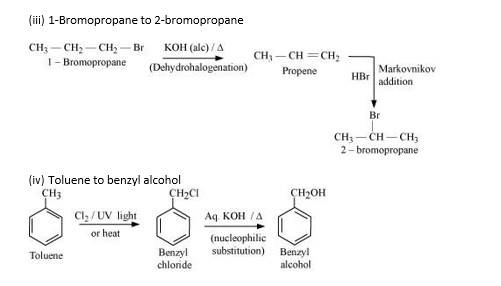
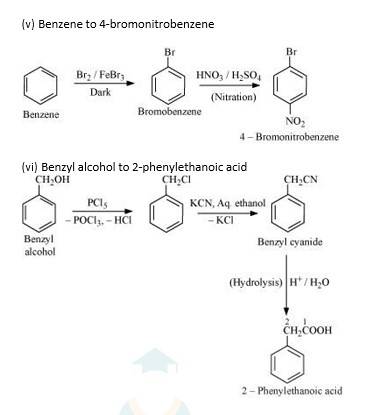
10.19 How the following conversions can be carried out? (i) Propene to propan-1-ol (ii) Ethanol to but-1-yne (iii) 1-Bromopropane to 2-bromopropane (iv) Toluene to benzyl alcohol (v) Benzene to 4-bromonitrobenzene (vi) Benzyl alcohol to 2-phenylethanoic acid (vii) Ethanol to propanenitrile (viii) Aniline to chlorobenzene (ix) 2-Chlorobutane to 3, 4-dimethylhexane (x) 2-Methyl-1-propene to 2-chloro-2-methylpropane (xi) Ethyl chloride to propanoic acid (xii) But-1-ene to n-butyliodide (xiii) 2-Chloropropane to 1-propanol (xiv) Isopropyl alcohol to iodoform (xv) Chlorobenzene to p-nitrophenol (xvi) 2-Bromopropane to 1-bromopropane (xvii) Chloroethane to butane (xviii) Benzene to diphenyl (xix) Tert-Butyl bromide to isobutyl bromide (xx) Aniline to phenylisocyanide
(i) Propene to propan-1-ol
It is an antimarkovnikoff reaction in which under the presence of a peroxide an alkene undergoes substitution, wherein the halo group is attached to that carbon which has least no. Of alkyl groups attached to it.
10.31 Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
There are two primary alkyl halides having the formulaC4H9Br, They are n - butyl bromide and isobutyl bromide.
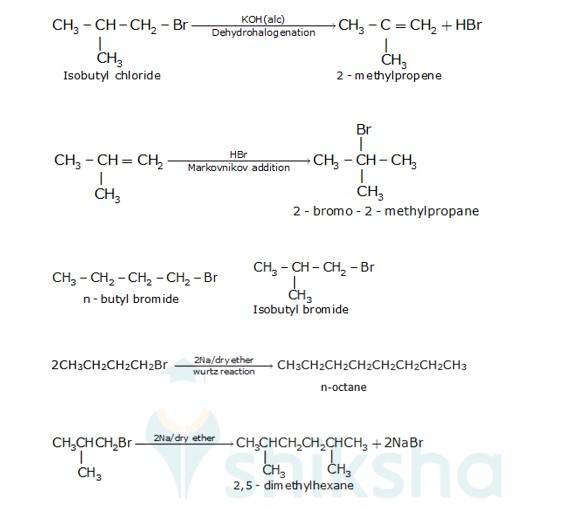
Therefore, compound (a) is either n-butyl bromide or isobutyl bromide.
Now, compound (a) reacts with Na metal to give compound (b) of molecular formula, C18H18 which is different from the compound formed when n-butyl bromide reacts with Na metal.
Hence, compound (a) must be isobutyl bromide. Thus, compound (d) is 2, 5-dimethylhexane.
It is given that compound (a) reacts with alcoholic KOH to give compound (b). Hence, compound (b) is 2- methylpropene.
Also, compound (b) reacts with HBr to give compound (c) which is an isomer of (a). Hence, compound (c) is 2-bromo-2-methylpropane.
10.13 Which one of the following has the highest dipole moment?
(i) CH2Cl 2 (ii) CHCl 3 (iii) CCl4
Dipole moment of a molecule depends on the electronegativity difference between atoms bonded covalently and geometry of the molecule (how far the atoms are from each other). Dipole moment is important to understand the polarity of a molecule.
The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:-
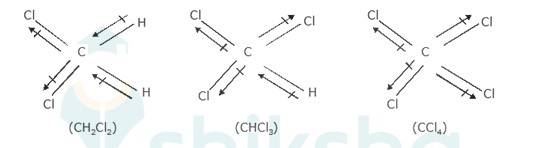
CCl4 being symmetrical has zero dipole moment. In CHCl3, the resultant of the two C-Cl dipole moments is opposed by the resultant of C-H and C-Cl bonds. Since the dipole Moment of latter resultant is expected to be smaller than the former, CHCl3 has a finite dipole moment (1.03D)
In CH2Cl2, the resultant of two C-Cl dipole moments is reinforced by resultant of two C-H dipoles. Therefore, CH2Cl2 (1.62D) has a dipole moment higher than that of CHCl3. Thus, CH2Cl2 has the highest dipole moment.
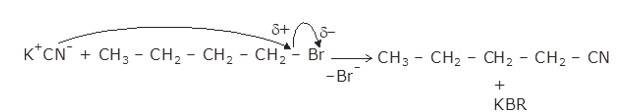
10.25 Write the mechanism of the following reaction:
The given reaction is a nucleophillic reaction:

The given reaction is an SN2 reaction. In this reaction, CN acts as the stronger nucleophile and attacks the carbon atom to which Br is attached in nBuBr.
And, CN- ion is an ambident nucleophile and can attack through both C and N. In this case, it attacks through the C-atom.
The mechanism is shown below:
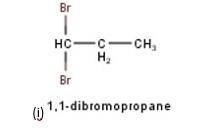
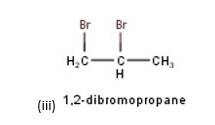
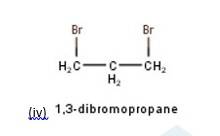
10.3 Write structures of different dihalogen derivatives of propane.
10.3 There are four different dihalogen derivatives of propane. The structures of these derivatives are as shown below: -

10.32 What happens when (i) N-butyl chloride is treated with alcoholic KOH (ii) Bromobenzene is treated with Mg in the presence of dry ether (iii) Chlorobenzene is subjected to hydrolysis (iv) Ethyl chloride is treated with aqueous KOH (v) Methyl bromide is treated with sodium in the presence of dry ether (vi) Methyl chloride is treated with KCN?
This is a classic question from the chapter Haloalkanes and Haloarenes. Let us solve each one as follows:
(1) When n - butyl chloride is treated with alcoholic KOH, the formation of but - l - ene takes place. This reaction is a dehydrohalogenation reaction.
When N-butyl chloride or 1-chlorobutane is treated with alcoholic potassium hydroxide (KOH), elimination reaction takes place. This leads to alkene formation. 1-butene is the major product here.
(ii) When Bromobenzene is treated with Mg in the presence of dry ether, it undergoes a reaction to produce phenylmagnesium bromide which is the Grignard reagent. This reagent is very reactive organomagnesium compound. Ether solvent is important since it produces a complex with Grignard reagent.
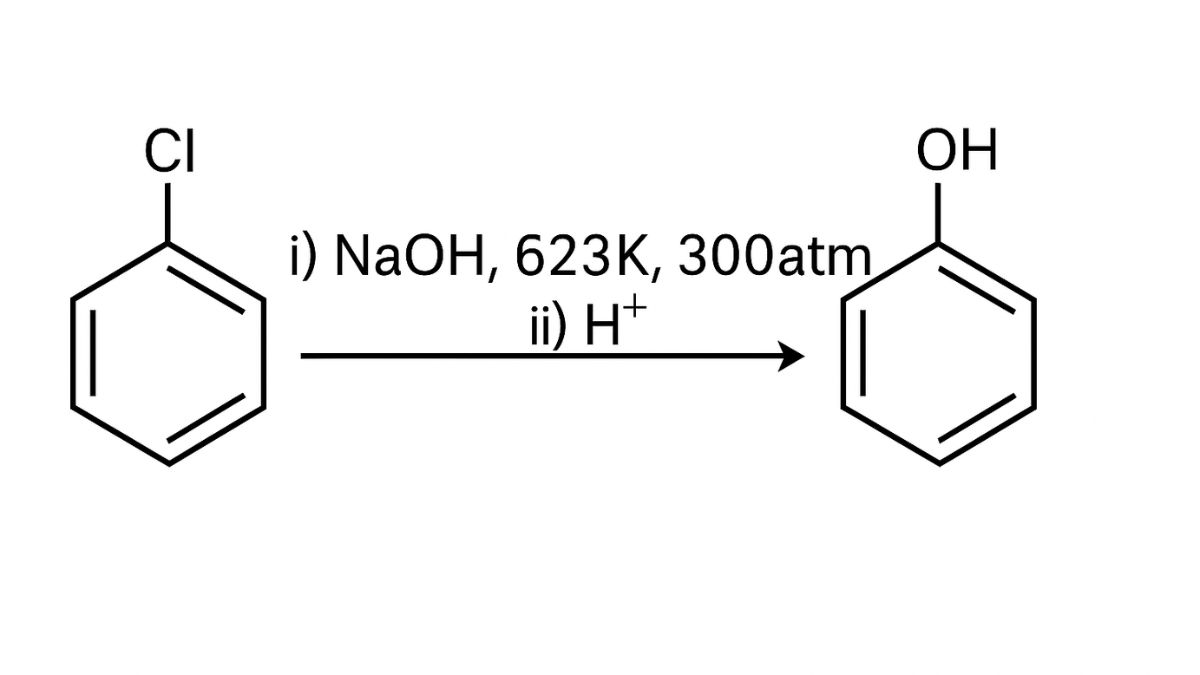
(iii) Chlorobenzene is aryl halide does not easily react with dilute adequate bases or water. However, under extreme conditions like high temperature and pressure and in the presence of strong base/acid catalyst, chlorobenzene undergoes hydrolyosis to form phenol.
10.11 Give the IUPAC names of the following compounds:
(i) CH3CH(Cl)CH(Br)CH3 (ii) CHF2CBrClF (iii) ClCH2C≡CCH2Br (iv) (CCl3 ) 3CCl (v) CH3C(p-ClC6H4 ) 2CH(Br)CH3 (vi) (CH3 ) 3CCH=CClC6H4 I-p
CH3CH (Cl)CH (Br)CH3: 2-Bromo-3-chlorobutane
- Write the name of side substituent according to the alphabetical order. Example: bromo is written before chloro as B comes before
- Always use a hyphen {-} between a number and a Since Br is attached to 2 position and Cl is attached to third position i.e. it is written as 2-Bromo and 3-chloro.
- Lastly, write the name of the longest hydrocarbon chain e. butane of 4 carbons.
CHF2CBrClF :1-bromo-1-chloro-1,2,2-trifluoroethane
- While writing the IUPAC name, write the name of side substituent according to the alphabetical Eg: bromo is written before chloro and fluoro as B comes before C and F.
- Always use a hyphen {-} between a number and a Since Br is attached to first carbon and Cl Is also attached to first carbon i.e. it is written as 1-Bromo and 1-chloro.
- Since there are 3 F present so it is written as
- And at the last write the name of the longest hydrocarbon chain e. ethane of 2 carbons.
ClCHC≡CCH2Br: 1-bromo-4-chlorobut-2-yne
- While writing the IUPAC name, write the name of side substituent according to the alphabetical That is why bromo is written before chloro as B comes before C.
- Always use a hyphen {-} between a number and a Since Br is attached to 1 position and Cl isattached to fourthposition i.e. it is written as 1-Bromo and 4-chloro.
- Also there is a presence of triple bond at the second carbon so the suffix used for triple bond is “yne” along with the position of the bond i.e. but-2-yne
(CCl3)3CCl
2- (trichloromethyl)-1,1,1,2,3,3,3-heptachloropropane
Explanation:
- While writing the IUPAC name, write the name of side substituent according to the alphabetical
- Always use a hyphen {-} between a number and a
- Because of the presence of 3 CCl3 group tri is used as a
- Since 7 Cl atoms are present in all therefore hepta is used a prefix with the position of chlorine. And at the last write the name of the longest hydrocarbon chain e. propane)
CH3C (p-ClC6H4)2CH (Br)CH3
2-bromo-3,3-bis- (4-chlorophenyl)butane
Explanation:
- While writing the IUPAC name, write the name of side substituent according to the alphabetical
- Always use a hyphen {-} between a number and a
- Because of the presence of 2 (p-ClC6H2) group bis is used as a prefix instead of di because C6H4 contains itself contains numbers 6 &
- And at the last write the name of the longest hydrocarbon chain e. butane)
(CH3)3CCH=CClC6H4I-p
1-chloro-1- (4-iodophenyl)-3,3-dimethylbut-1-ene
Explanation:
- While writing the IUPAC name, write the name of side substituent according to the alphabetical
- Always use a hyphen {-} between a number and a
- Because of the presence of 2 methyl group di is used as a
- And the presence of double bond has the ending ‘ene’ and the presence of double bond at the first position is written as but-1-ene.)
10.22 Explain why (i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) Alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous conditions?
Let us explain each question from Haloalkane and Haloarenes chapter one by one. Students who are currently in school and plan to take the CBSE board exam for class 12th soon, need to prepare all these questions. Let us get started.
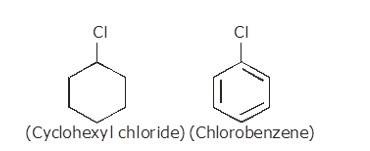
(i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Sp2 hybrid carbon (s-character=33.33%) in chlorobenzene is more electronegative than a sp3-hybrid carbon (s-character=25%) in cyclohexylchloride, due to greater s-character. Thus, Carbon atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
Chlorine atom in chlorobenzene is attached to the benzene ring. Here, the carbon-chlorine bond is polar. However, electron delocalization is possible in the benzene ring. Due to the electron delocalization, the overall dipole moment reduced since the polarity is distributed over the entire ring structure.
On the other hand, the chlorine atom in cyclohexyl chloride is attached to a saturated carbon atom in the cyclohexane ring. Because of this, there is no electron delocalization in the saturated structure. Hence, the dipole moment is more localized and stronger as compared to chlorobenzene.
(ii) Alkyl halides, though polar, are immiscible with water.
Alkyl halides are polar because of the difference in electronegativity between carbon and halogen atoms. Here, there is not enough polarity to make them soluble in water.
The alkyl part of molecule is non-polar as well as hydrophobic in nature. This portion is large enough to dominate the solubility characteristics of the molecule. The entire molecule becomes insoluble in water due to this.
Water molecules create extensive hydrogen bonds with each other. However, Alkyl halides cannot form hydrogen bonds with water, which reduces their solubility in water. Haloalkane and Haloarene NCERT excercise discusses this question in further detail.
(iii) Grignard reagents should be prepared under anhydrous conditions.
Grignard reagents are very reactive organometallic compounds and they react readily with water to produce hydrocarbons. This destroys the Grignard reagent and makes it ineffective for its original purpose.
The reaction with water not only destroy Grignard reagent but also it produces by products that can complicate the synthetic pathway.
Preparing Grignard reagents under anhydrous conditions makes sure that they remain stable and effective for use in further reactions. Moisture-free conditions are important for the formation and utilization of reagents.
R-Mg-X + H-OH → R-H + Mg (OH)X
Do note that questions based on such equations from organic chemistry are also be asked in the CUET exam which makes it important for students to remember it.
Thus, Grignard reagents must be prepared under anhydrous conditions.
10.19 Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene: (i) 1-Bromo-1-methylcyclohexane (ii) 2-Chloro-2-methylbutane (iii) 2,2,3-Trimethyl-3-bromopentane
Carbon due to its chemical property of catenation, tetravalency and the ability to form stable covalent bonds form hydrocarbon and halo-alkanes easily.
The process of removal of hydrogen and halogen from adjacent carbon atoms of haloalkens is called dehydrohalogenation. NaOEt ( Sodium ethoxide) used in Dehydrohalogenation. It is a strong base and helps remove a β-hydrogen from the haloalkane.
The chemical reaction for
Reaction:
R–CH2 –CHX–R′+NaOEt ethanol > R–CH=CHR’+NaX+EtOH
(i) 1-Bromo-1-methylcyclohexane:
Br
|
CH3–C1–cyclohexane + NaOEt/EtOH → CH3–C=cyclohexane (double bond at C1-C2) + NaBr + EtOH
Major Product: 1-Methylcyclohexene
(ii) 2-Chloro-2-methylbutane
CH3
|
CH3–C–CH2–CH3 + NaOEt/EtOH → CH3–C=CH–CH3 + NaCl + EtOH
| ||
Cl CH3
Major Product: 2-Methyl-2-butene (major), 2-Methyl-1-butene (minor)
(iii) 2,2,3-Trimethyl-3-bromopentane
CH3 CH3
| |
CH3–C–C–CH2–CH3 + NaOEt/EtOH → CH3–C=C–CH2–CH3 + NaBr + EtOH
| | ||
CH3 Br CH3
Major Product: 2,2,3-Trimethyl-2-pentene
10.24 Write the structure of the major organic product in each of the following reactions:
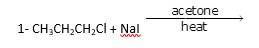
It is a simple substitution reaction with Cl being replaced by iodide ion.
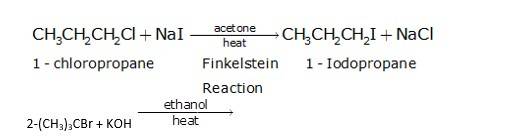
Since ethanol is a alcohol, so in presence of alcoholic KOH, alkyl chloride undergo elimination reaction, which results in the removal of proton and being substituted by a bromide ion.
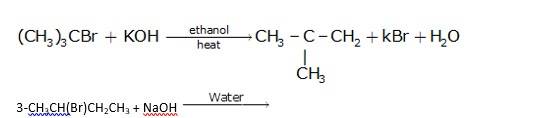
It is also a simple substitution reaction in which under the presence of aqueous NaOH, bromide ion is replaced by hydroxide ion as it is a better leaving group than hydroxide ion.
It is a simple substitution reaction in which a better leaving group leaves and is being substituted by an ion.
CH3CH2Br + KCN - CH3CH2CN + kBr
5-C6H5ONa + C2H5Cl
It is a simple substitution reaction in which a better leaving group leaves and is being substituted by an ion.
C6H5ONa + C2H5Cl? C6H5 – O – C2H5 + NaCl
6-CH3CH2CH2OH + SOCl2
This reaction is called as wurtz reaction in which in which clubbing together of a alkyl halide group with a sodium or potassium salt of an ether group.
7-CH3CH2CH = CH2+ HBr
It is an antimarkovnikoff reaction in which under presence of a peroxide an alkene undergoes substitution, wherein the halo group is attached to that carbon which has least no. Of alkyl groups attached to it.
8-CH3CH = C (CH3)2 + HBr
It is a markovnikoff reaction in which an alkene undergoes substitution reaction with hydrohalide, wherein a halo group is attached to that carbon atom which has the largest no. Of alkyl groups attached to it.
10.21 How will you bring about the following conversions? (i) Ethanol to but-1-yne (ii) Ethane to bromoethene (iii) Propene to 1-nitropropane (iv) Toluene to benzyl alcohol (v) Propene to propyne (vi) Ethanol to ethyl fluoride (vii) Bromomethane to propanone (viii) But-1-ene to but-2-ene (ix) 1- Chlorobutane to n-octane (x) Benzene to biphenyl.(Advanced)
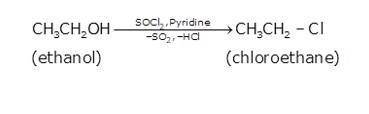
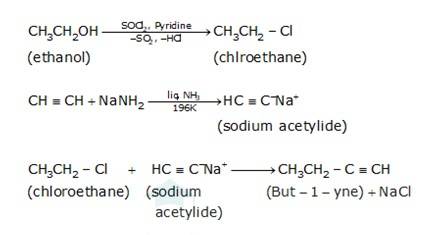
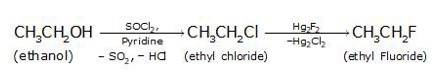
(i) Since the conversion of ethanol to but-1-yne involves the addition the two extra carbons that is why the conversion is carried out in 3 steps.
- In the first step ethanol is treated with thionyl chloride (SOCl2) in the presence of pyridine to give chloroethane
- In the second step the acetylene is treated with sodamide(NaNH2) in the presence of liquid ammonia to give sodium acetylide
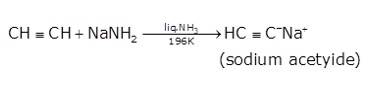
- The last step involves the reaction between chloroethane and sodium acetylide to give the final product as the But-1-yne and NaCl as the by-product.
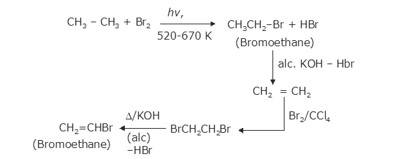
- The bromination of ethane (Br2 in the presence of light at 520-670K) gives bromoethane which on further treatment with alcoholic KOH results in the alkene called ethane which again on bromination with Br2/CCl4 gives dibromide(vicinal bromide) called 1,2-dibromoethane which on treatment with alcoholic KOH gives bromoethene as the final product.
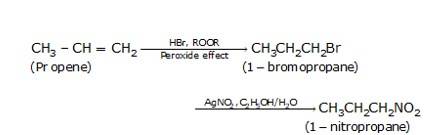
- Propene on treatment with HBr in the presence of peroxide (anti-markovnikoff’s rule which states the negative part e. Br- goes to the carbon which has more number of hydrogens and positive part i.e. H+ goes to the carbon which has lesser number of hydrogens) gives 1-bromopropane which on treatment with silver nitrite in the presence of alcohol or water gives 1-nitropropane as the final product.
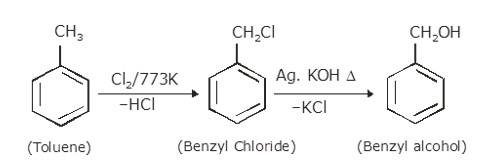
- Chlorination of toluene (Cl2 at 773K) gives benzyl chloride with the removal of HCl. Benzyl Chloride on further treatment with dilute KOH in the presence of heat gives benzyl alcohol as the final (dilute KOH removes Cl from the benzyl chloride and replaces it by OH)
- Propene is treated with Br2/CCl4e. bromination of alkene takes place to give dibromides, in this case 1,2-dibromopropane which on further treatment with alcoholic KOH gives propyne.(two times removal of KBr)
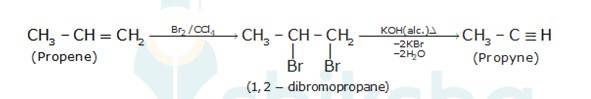
- Ethanol in treatment with thionyl chloride(SOCl2) in the presence of pyridine gives ethyl chloride with the evolution of SO2 The ethyl chloride on treatment with mercury fluoride gives ethyl fluoride (the Cl of ethyl chloride is being replaced by F from Hg2F2).
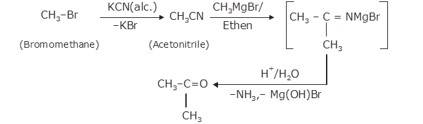
- Bromoethane on treatment with alcoholic KCN forms Acetonitrile with the removal of KBr. CN is added in the case where there is a need to increase the number of Further the acetonitrile is treated with CH3MgBr(Grignard reagent) in the presence of ether, the intermediate so formed is hydrolysed, and the product formation takes place i.e. Propanone and NH3 is obtained as the by- product.
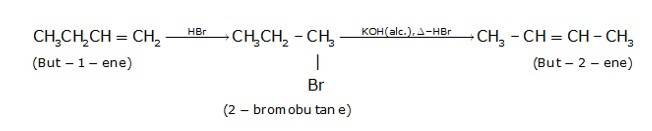
- But-1-ene reacts with HBr to form 2-bromobutane(acc. To markovnikoff’s rule) and further 2- bromobutane is treated with alcoholic KOH in the presence of heat to form But-2-ene with the removal of HBr.(alcoholic KOH removes H from the carbon which has less number of hydrogens just next to the carbon to which halide is attached and gives the major product e. Saytzeff rule)
- The conversion of 1-chlorobutane to n-octane in the presence of Na metal and dry ether is called Wurtz Two equivalent Chlorobutane reacts to give n-octane as a final product and NaCl as the by-product.
The conversion of benzene to biphenyl using Na and dry ether is called FITTIG reaction.
Firstly, the benzene is treated with Br2/FeBr3 results in the formation of bromobenzene. It is an electrophilic substitution. Further the bromobenzene is treated with Na metal in presence of dry ether to form biphenyl as the final product and NaBr as the by-product.
If instead of Na metal, bromobenzene is treated with Cu metal then the reaction is called Ullmann’s reaction. The final product obtained in this reaction will also be biphenyl.
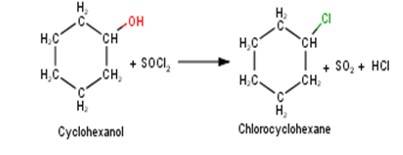
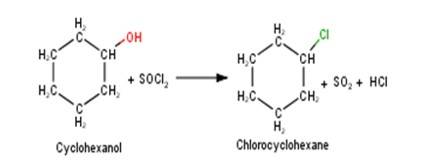
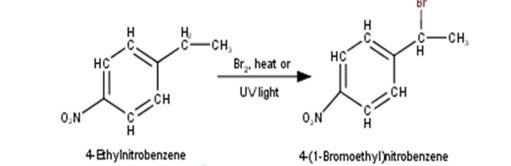
10.5 Draw the structures of major monohalo products in each of the following reactions
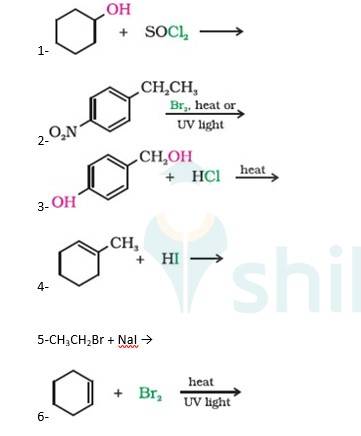
A 10.5 1. Cyclohexanol will react with thionyl chloride to form Chlorocyclohexane with the evolution of sulphur dioxide and hydrogen chloride gas as shown below :
2. 4-Ethylnitrobenzene will undergo benzylic bromination in presence of heat or light. Now, as benzylic radicals are more stable therefore benzylic hydrogen is abstracted. Hence, the reaction yields 4-(1- Bromoethyl)nitrobenzene as a product
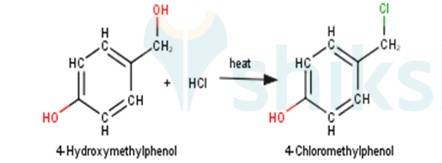
3. 4-Hydroxymethylphenol will react with hydrochloric acid under thermal conditions to yield 4- Chloromethylphenol a shown in the reaction below:
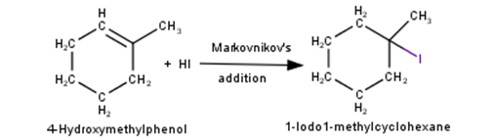
4. 1-Methylcyclohexene will react with hydrogen iodide via markovnikov’s addition mechanism to give 1- Iodo1-methylcyclohexane as shown in the reaction below: -
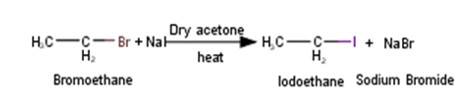
5.Bromoethane will react with sodium iodide in a Finkelstein reaction manner to yield Iodoethane in presence of dry acetone and heat as shown in the reaction below :
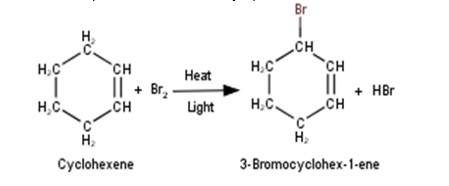
6. Cyclohexene reacts with bromine in presence of heat and light to undergo allylic bromination to yield 3-bromocyclohex-1-ene as the major product as shown in the reaction below :
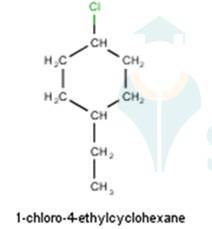
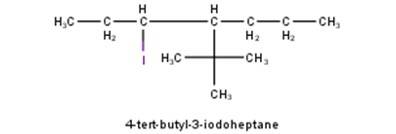
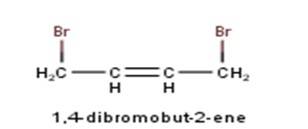
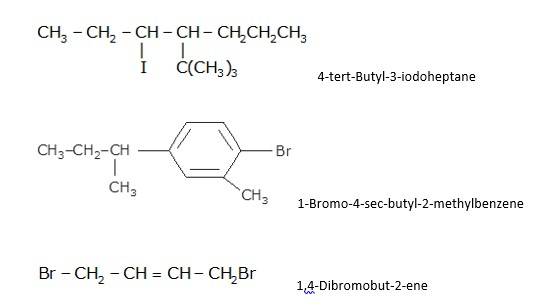
10.1 Write structures of the following compounds: (i) 2-Chloro-3-methylpentane (ii) 1-Chloro-4- ethylcyclohexane (iii) 4-tert. Butyl-3-iodoheptane (iv) 1,4-Dibromobut-2-ene (v) 1-Bromo-4-sec. butyl- 2-methylbenzene.
10.1 From the name of the compound it is clear that the parent ring is pentane and chloro and methyl groups are attached in the straight chain at 2nd and 5th position respectively. Hence, the structure is as follows:
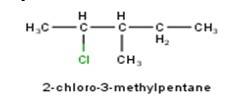
From the name of the compound given it is clear that the parent group is hexane with 2 attachments namely chloro and ethyl groups at 1 and 4 positions respectively. Hence, the structure is as follows: -
From the name of the compound given it is clear that the parent group is heptane with tertiary butyl and iodine groups attached at 4 and 3 positions respectively. Hence, the structure is as follows: -
From the name of the compound given it is clear that the parent group is an alkene with the unsaturation present in between 2nd and 3rd carbon and 2 bromine atoms attached at 1st and 4th carbon. Hence, the structure is as follows : -
From the name of the compound given it is clear that the parent group is benzene with bromine group attached at 1st carbon, secondary butyl group attached at 4th carbon and a methyl group attached at 2nd carbon. Hence, the structure is a follows : -
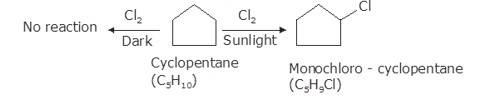
10.14 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
As per the molecular formula , one thing can be confirmed; the hydrogen is either cycloalkane or it is an alkene. However, alkenes readily react with chlorine in even dark because of the double bond. Therefore, it is possible that the hydrocarbon is cycloalkane.
Now, let us consider the reactivity. Cycloalkanes are the saturated hydrocarbons with no double bonds. These do not react with Chlore in dark because such a reaction needs UV light for initiating the process of free radical substitution. Haloalkane and Haloarenes NCERT solutions cover this as well as other questions in further detail.
In bright sunlight, the UV light starts free radical substitution reaction. In cyclopentane, the substitution will result in single monochloro product because all hydrogen atoms are equivalent in the molecule. Therefore, by replacing any hydrogen with chlorine atom will result again in the same product i.e. This confirms that the hydrocarbon is cyclopentane.
10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
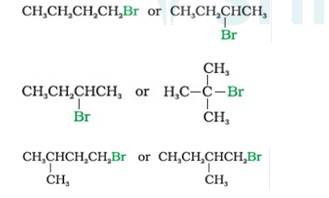
In SN2 Reaction, the product will be formed in a single step with no intermediates. Nucleophile attack will be easier for simple halides than hindered haloalkanes. Therefore,

- Bromobutane (1°)reacts faster than 2-Bromobutane (2°)
- 2-Bromobutane (2°) reacts faster than 2-Bromo-2-methylpropane or tert-Butyl bromide (3°) 1-Bromo-3-methyl butane reacts faster than the 1-Bromo-2-methyl bu
- tane as the former is less hindered with respect to leaving halide than the later which is more hindered comparatively
10.2 Why is sulphuric acid not used during the reaction of alcohols with KI?
In the presence of sulphuric acid (H2SO4), KI produces HI as follows : -
2KI + H2SO4 → 2KHSO4 + 2KI
Since H2SO4 is an oxidising agent, it oxidises HI (produced in the reaction to I2)
2HI + H2SO4 → I2 + SO2 +;
As a result, the reaction between an alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidising acid such as H3PO4 is used in the reaction to get the desired product.
A few things you can remember here, while solving NCERT Solutions for Haloalkanes And Haloarenes.
Sulphuric acid is a powerful oxidising agent. We know this because the sulphur atom in sulphuric acid is in its highest possible oxidation state (+6). It can only be reduced. And it can readily accept electrons from other substances.
It oxidises the necessary intermediate, hydrogen iodide (HI), to iodine (I2). We know this because iodide ions are strong reducing agents, and they can simply give up electrons. So when sulphuric acid accepts electrons, it can convert the iodide to elemental iodine.
The alcohol cannot be converted to alkyl iodide. The reason for this is that the side reaction consumes the iodide ions necessary to act as a nucleophile and replace the alcohol's -OH group. This is the desired alkyl iodide.
A non-oxidising acid like phosphoric acid (H3PO4) is used instead to produce the desired alkyl iodide. Phosphoric acid is a weaker oxidising agent than sulphuric acid and does not react with the iodide ions. That allows the intended reaction with the alcohol to proceed.
10.30 The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
An aqueous solution, KOH almost completely ionizes to give OH-ions. OH- ion is a strong nucleophile (see the imp. note below), which leads the alkyl chloride to undergo a nucleophilic substitution reaction to form alcohol.
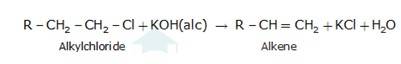
On the other hand, an alcoholic solution of KOH contains alkoxide (RO-) ion, it is a strong base. Thus, it can abstract a hydrogen ion from the beta-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.OH- ion is a much weaker base than RO- ion. Also, OH- ion is highly solvated (because more energy is released on solvation)in an aqueous solution and as a result, the basic character of OH-ion decreases.
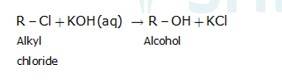
Therefore, it cannot abstract a hydrogen from the beta -carbon.
The concept used hydroxide ion is a strong nucleophile but weaker base than -OR group.
10.8 In the following pairs of halogen compounds, which compound undergoes faster SN 1 reaction?
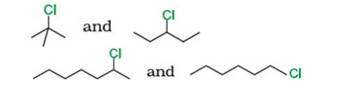

Reason: SN1 occurs in two steps. In the first step, carbocation forms. The rate of reaction in SN1 depends upon the stability of the carbocation, greater the stability faster the reaction. Tertiary (3°) carbocation is more stable than secondary (2°), which is further stable than primary (1°)
By using this
- Tert-Butyl Chloride (3°) reacts faster than 3-Chloropentane (2°)
- 2-Chloro heptane (2°) is more reactive than 1-Chloro hexane (1°)
10.27 Out of C6H5CH2Cl and C6H5CHClC6H5 , which is more easily hydrolysed by aqueous KOH.
Hydrolysis by KOH results in the formation of the carbocation. Those compounds which leads to the formation of stable carbocation are easily hydrolysed.C6H5CH2Cl leads to formation of 1°- carbocation, while C6H5CHClC6H5 forms 2°-carbocation, which is more stable than 1°-carbocation. Hence C6H5CHClC6H5, is hydrolyzed more easily than C6H5CH2Cl by aqueous KOH.
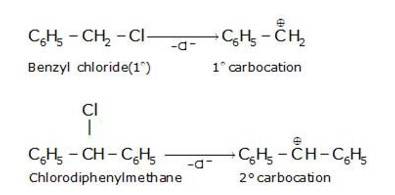
10.10 Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: (i) (CH3) 2CHCH(Cl)CH3 (ii) CH3CH2CH(CH3 )CH(C2H5 )Cl (iii) CH3CH2C(CH3 ) 2CH2 I (iv) (CH3 ) 3CCH2CH(Br)C6H5 (v) CH3CH(CH3 )CH(Br)CH3 (vi) CH3C(C2H5 ) 2CH2Br (vii) CH3C(Cl)(C2H5 )CH2CH3 (viii) CH3CH=C(Cl)CH2CH(CH3 ) 2 (ix) CH3CH=CHC(Br)(CH3 ) 2 (x) p-ClC6H4CH2CH(CH3 )2 (xi) m-ClCH2C6H4CH2C(CH3 )3 (xii) o-Br-C6H4CH(CH3 )CH2CH3
(i) 2-chloro-3-methylbutane, (2? alkyl halide)
Explanation: The Cl atom is attached with two alkyl groups therefore 2? alkyl halide.
(ii) 3-chloro-4-methylhexane (2? alkyl halide)
Explanation: The Cl atom is attached with two alkyl groups therefore 2? alkyl halide.
(iii) 1-iodo-2,2-dimethylbutane (1? alkyl halide)
Explanation: The atom I is attached with one alkyl group therefore 1? alkyl halide.
(iv) 1-bromo-3,3-dimethyl-1-phenylbutane (2? benzylic halide)
Explanation: The atom Br is attached with two alkyl groups with the benzene group therefore 2? benzylic halide.
(v) 2-bromo-3-methylbutane (2? alkyl halide)
Explanation: The atom Br is attached with two alkyl groups therefore 2? alkyl halide.
(vi) 1-bromo-2-ethyl-2-methylbutane (1? alkyl halide)
Explanation: The atom Br is attached with one alkyl group therefore 1? alkyl halide.)
(vii) 3-chloro-3-methylpentane (3? alkyl halide)
Explanation: The atom Cl is attached with three alkyl groups therefore 3? alkyl halide.
(viii) 3-chloro-5-methylhex-2-ene (vinylic halide)
Explanation: The atom Cl is attached with the double bond i.e. at vinylic position ∴ vinylic halide.)
(ix) 4-bromo-4-methylpent-2-ene (allylic halide)
Explanation: The Br atom is attached with the carbon next to the double bond i.e. at allylic position therefore allylic halide.
(x) 1-chloro-4- (2-methylpropyl)benzene (aryl halide)
Explanation: The Cl atom is attached with the benzene i.e. at aryl position therefore aryl halide.
(xi) 1-chloro-3- (2,2-dimethylpropyl)benzene (1? benzylic halide)
Explanation: The Cl is attached with one alkyl group at the benzene carbon therefore 1? benzylic halide.
(xii) 1-bromo-2- (1-methylpropyl)benzene (aryl halide)
Explanation: The Br is attached with the benzene i.e. at aryl position therefore aryl halide.
10.15 Write the isomers of the compound having formula C4H9Br.
Double bond equivalent is used to find the level of unsaturations present in an organic molecule.
DBE = C +1-H/2+X/2-N/2
Where C= number of carbon atoms present H=number of hydrogen atoms present N=number of nitrogen atoms present X=number of halogen atoms present Double bond equivalent (DBE) for C4H9Br
= 4+1-9/2+1/2
=0
So none of the isomers has a ring or unsaturation, so the isomers are positions or chain isomers as shown below in the table:

10.17 What are ambident nucleophiles? Explain with an example.
Ambident Nucleophiles are those nucleophilies which can attack through different sites. For example:-cyanide ions are a resonance hybrid of the following two structures:

It can attack through carbon to form cyanide and through N to form is O cyanide. Example: NO2, NO3 - etc.
10.23 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
(i) Freon 12:- the chlorofluorocarbon compounds of methane and ethane are collectively known as freons. They are extremely stable, unreactive, non-toxic and non-corrosive. Example of Freon 12 is CCl2F2. It is mainly used in refrigeration and eventually makes its way into the atmosphere where it diffuses unchanged into the stratosphere. In stratosphere, Freon is able to initiate the radical chain reactions that can upset the natural ozone balance.
- DDT: - it stands for p, p'-Dichlorodiphenyltrichloroethane (DDT). It is mainly used as an
- Iodoform: - it was earlier used as an antiseptic but the antiseptic properties are due the liberation of free iodine and not due to the iodoform itself. Due to its objectionable smell, it has been replaced by other formulations containing iodine.
- Carbon tetrachloride (CCl4):- it is used as an industrial solvent for oils, fats, resins etc. and also in dry Used as a cleaning fluid both in industries, as a degreasing agent and in the home, as a spot remover and as a fire extinguisher.
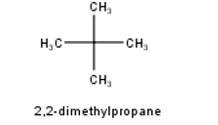
10.4 Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields (i) A single monochloride. (ii) Three isomeric monochlorides. (iii) Four isomeric monochlorides
To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because of the fact that replacement of any H-atom leads to the formation of the same product. Therefore the isomer is 2,2-dimethylpropane.
To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms. Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane as shown below : -
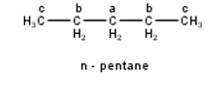
To have four isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain four different types of H-atoms in it. Therefore, the required isomer is 2-methylbutane. It can be observed that there are four different types of H-atoms labelled as a, b, c, and d in 2-methylbutane as shown below: -
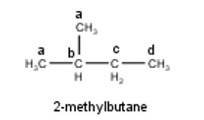
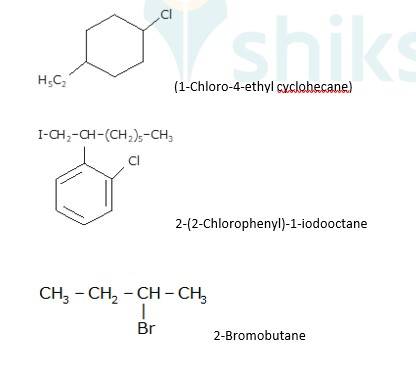
10.12 Write the structures of the following organic halogen compounds. (i) 2-Chloro-3-methylpentane (ii) p-Bromochlorobenzene (iii) 1-Chloro-4-ethylcyclohexane (iv) 2-(2-Chlorophenyl)-1-iodooctane (v) 2-Bromobutane (vi) 4-tert-Butyl-3-iodoheptane (vii) 1-Bromo-4-sec-butyl-2-methylbenzene (viii) 1,4- Dibromobut-2-ene
- Write the longest chain first e. in this case it is ‘pentane’ consisting of 5 carbons.
- Now, place the substituents at their respective place. For eg. Chlorine(Cl) at second carbon and methyl(CH3) at third carbon.
- Write the longest chain first i.e. in this case it is ‘benzene’ a cyclic structure consisting of 6 carbons
with 3 double bonds.
Now, place the substituent Bromine(Br) at the para position i.e. at the fourth carbon.
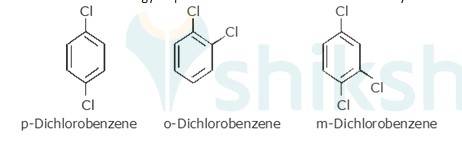
10.28 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
p-Dichlorobenzene is more symmetrical than o-and m-isomers. Because it fits more closely and easily in the crystal lattice than o-and m-isomers. Therefore, more energy is required to break the crystal lattice of p-dichlorobenzene. Therefore, p-dichlorobenzene is symmetrical and has a higher melting point and lower solubility than o-and m-isomers due to strong force of attraction in crystal.
Therefore more energy required to break lattice and will not easily be soluble.
10.6 Arrange each set of compounds in order of increasing boiling points. (i) Bromomethane, Bromoform, Chloromethane, Dibromomethane. (ii) 1-Chloropropane, Isopropyl chloride, 1- Chlorobutane.
The order of increasing boiling point is
- Chloromethane (CH3Cl)< Bromomethane (CH3Br) < Dibromomethane (CH2Br2)< Bromoform (CHBr3)
- Isopropyl chloride (C3H7Cl)< 1-Chloropropane (C3H7Cl) < 1-Chlorobutane (C4H9Cl)
Generally, boiling point increases with the molecular weight of the compound and decreases with the branching of the chain
Chemistry Ncert Solutions Class 12th Exam
Student Forum
Other Similar chapters for you
- Aldehydes, Ketones and Carboxylic Acids
- Chemistry in Everyday Life
- Polymers
- Surface Chemistry
- General Principles & Processes of Isolating Elemen
- P Block Elements
- Alcohol Phenol And Ethers
- Haloalkanes and Haloarenes
- Amines
- NCERT Chemistry 12th
- Biomolecules
- Coordination Compounds
- Block D and F Elements
- Chemical Kinetics
- Electrochemistry