
Biomolecules Class 12 covers complex biomolecules including proteins, carbohydrates, lipids and nucleic acids. We get the essential biomolecules such as carbohydrates and proteins from our food. Other simple molecules, such as vitamins and mineral salts, contribute significantly to the functions of living organisms.
Biomolecules Chemistry Class 12 explains the characteristics of proteins, nucleic acids, carbohydrates and hormones. It classifies proteins, vitamins, nucleic acids, and carbohydrates on the basis of their structures, differentiates between DNA and RNA, and describes the role of biochemicals in biosystems. Biomolecules Class 12 chemistry is an important chapter for the CBSE Board exam and other competitive exams like NEET and JEE Main. Students must practice the Biomolecules NCERT and score well in their examinations.
If you are looking for the topic-wise Class 12 Chemistry notes with free PDF and solved questions, you must read here.
- Quick Overview of NCERT Solutions for Class 12 Biomolecules
- Class 12 Chemistry Chapter 10 Biomolecules: Key Topics and Important Reactions
- NCERT Chemistry Class 12th Biomolecules Solution PDF: Free PDF Download
- NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules: Intext Solutions
- Benefit of using NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
Quick Overview of NCERT Solutions for Class 12 Biomolecules
See below the key highlights of the Biomolecules chapter:
- Carbohydrates are classified into three groups - monosaccharides, disaccharides and polysaccharides.
- Glucose is obtained from starch and it is the most important source of energy in mammals.
- Proteins are the polymers of the 20 different amino acids, which are linked by peptide bonds.
- The chapter covers the 10 essential amino acids. These should be provided by the diet as they cannot be synthesized by our body.
- It covers the secondary or tertiary structure of proteins and the denaturation of proteins.
- The chapter covers the vitamins, which are classified as the fat-soluble A, D, E, and K and the water-soluble B and C groups.
- There are two types of nucleic acids - DNA and RNA.
For comprehensive notes of Class 12 Physics, Chemistry & Maths, the students need to click on NCERT Class 12 Notes.
Class 12 Chemistry Chapter 10 Biomolecules: Key Topics and Important Reactions
Biomolecules Class 12 is an important chapter of Chemistry. The main topics on which the students should focus for the entrance exam include carbohydrates, nucleic acids, proteins, and enzymes. Also, on the properties, structures, and functions of carbohydrates, proteins, amino acids, lipids, and nucleic acids. See the table below for the topics covered in the Biomolecules chapter:
| Exercise | Topics Covered |
|---|---|
| 10.1 | Carbohydrates |
| 10.2 | Proteins |
| 10.3 | Enzymes |
| 10.4 | Vitamins |
| 10.5 | Nucleic Acids |
| 10.6 | Hormones |
Class 12 Biomolecules Weightage in NEET, JEE Main Exams
| Exam | Number of Questions | Weightage |
|---|---|---|
| NEET | few questions, often around 6 | 10-15% |
| JEE Main | 3-4 questions | 8-9% |
Important Reactions of Class 12 Chemistry Biomolecules
- Reactions of Carbohydrates
Reduction of Aldoses and Ketoses
-
-
Glucose + H₂ → Sorbitol (CH₂OH-(CHOH)₄-CH₂OH)
-
(Using reducing agents like NaBH₄ or H₂/Ni)
-
Oxidation Reactions
-
-
Mild oxidation (Br₂):
-
Glucose → Gluconic acid
-
-
Strong oxidation (HNO₃):
-
Glucose → Saccharic acid
-
-
Osazone Formation
-
-
Glucose + Phenylhydrazine → Glucosazone
(Used to distinguish sugars)
-
Glycosidic Bond Formation
-
-
Monosaccharide + Alcohol → Glycoside
-
e.g., Glucose + Methanol → Methyl glucoside
-
-
Mutarotation
-
-
α-Glucose ⇌ β-Glucose (in aqueous solution)
(Change in optical rotation due to ring–open–ring conversion)
-
2. Reactions of Proteins (Amino Acids)
Zwitter Ion Formation
-
-
H₂N–CH(R)–COOH ⇌ ⁺H₃N–CH(R)–COO⁻
-
Peptide Bond Formation
-
-
Amino Acid₁ + Amino Acid₂ → Dipeptide + H₂O
-COOH + -NH₂ → -CO-NH- (Peptide bond)
-
Reaction with Ninhydrin (For detection of α-amino acids)
-
-
Amino acid + Ninhydrin → Purple/blue complex
-
Reaction with Formaldehyde (Used in titration)
-
-
NH₂ group reacts with HCHO to form methylene derivative
-
3. Reactions of Nucleic Acids
Hydrolysis of DNA/RNA
-
-
DNA/RNA + H₂O (acid or enzyme) → Sugar + Phosphoric acid + Nitrogenous base
-
Formation of Nucleoside
-
-
Sugar + Base → Nucleoside
-
Formation of Nucleotide
-
-
Nucleoside + Phosphate → Nucleotide
-
Phosphodiester Bond Formation
-
-
Nucleotide₁ + Nucleotide₂ → Dinucleotide + H₂O
-
4. Reactions of Enzymes
Catalysis Reaction
-
-
Substrate + Enzyme → Enzyme-substrate complex → Product + Enzyme
-
Temperature and pH Sensitivity
-
-
Enzymes are deactivated at high temperatures or extreme pH
-
5. Tests for Biomolecules
Fehling’s and Benedict’s Test (for Reducing Sugars)
-
-
Glucose + Fehling’s → Red ppt of Cu₂O
-
Biuret Test (for Proteins)
-
-
Protein + Biuret reagent → Violet color
-
Xanthoproteic Test (for Aromatic Amino Acids)
-
-
Protein + HNO₃ → Yellow color
-
NCERT Chemistry Class 12th Biomolecules Solution PDF: Free PDF Download
The link is given below for students to download the free Biomolecules NCERT PDF. These solutions are aligned with the NCERT syllabus. The NCERT solutions are prepared by the subject matter experts to help students get a high score in the CBSE Board exam and other entrance exams like NEET and JEE Main.
Download Here: NCERT Solution for Class XII Chemistry Biomolecules PDF
Read Here
| NCERT Notes for Class 11 & 12 | Class 12 Chemistry NCERT Solutions | NCERT Solutions Class 11 and 12 for Maths, Physics, Chemistry |
NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules: Intext Solutions
NCERT Chemistry Biomolecules intext solutions are provided here. Students can verify their answers using the biomolecules chemistry class 12 ncert solutions. Intext questions are based on the topics taught in class 12 Chemistry Ch 10 Biomolecules.
| Q 14.1 Glucose or sucrose is soluble in water but cyclohexane or benzene (simple six membered ring compounds) is insoluble in water. Explain. |
| Ans Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones). Structure of sucrose: As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water. This –H bonding is responsible for the solubility of glucose and sucrose in water. In case of cyclohexane or benzene (simple six-membered ring compounds) , they do not contain any – OH groups. Hence, they cannot undergo –H bonding with water and are insoluble. |
| Q 14.2 What are the expected products of hydrolysis of lactose? |
| Ans Lactose is a disaccharide carbohydrate (made up of two monosaccharide units) composed of β-D-galactose and β-D-glucose units. Hydrolysis breaks the glycosidic bond converting sucrose into β-D- galactose and β-D-glucose. NOTE: But however, this reaction is so slow that it takes years for the solution of sucrose to undergo negligible change. Hence an enzyme called sucrase is added to proceed rapidly. |
| Q 14.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose? |
| Ans D-glucose reacts with hydroxylamine (NH2OH) to form oxime due to the presence of the aldehyde functional group (-CHO). This is due to the cyclic structure of glucose which forms an open chain structure in an aqueous medium, which then reacts to give an oxime. But in case of pentaacetate of D-glucose, it does not form open chain structure in an aqueous medium so it does not react with NH2OH. |
| Q 14.4 The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain. |
| Ans Amino acids are organic compounds containing amine (basic) and carboxyl (acidic) functional group with a specific side chain. Both acidic and basic group are present in the same molecule. In, aqueous solution carboxyl group can lose a proton (H+) and amino group can accept a proton (H+) giving rise to the dipolar ion called as zwitter ion. Zwitter ion is shown below: |
Commonly asked questions
14.10 What are reducing sugars?
14.10
All those carbohydrates which reduce Fehling's solution to red precipitate of Cu2O or Tollen's reagent to metallic Ag is called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except Sucrose are reducing sugars.
14.18 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
14.18
Open structure of D-glucose could not explain the following reactions:
(i) Despite having the aldehyde group, glucose does not give 2,4 DNP test and Schiff's
(ii) Glucose does not react with sodium hydrogen sulphite to form addition
(iii) The absence of free –CHO group is shown when the penta-acetate of glucose does not react with hydroxyl
(iv) When glucose is heated with methanol in the presence of dry HCl gas, it forms two isomeric monomethyl derivatives known as? -D-glucoside and? -D-glucoside. Since only one molecule of methanol is used for the formation of methyl glucosided, these must be hemiacetals.
14.20 Define the following as related to proteins (i) Peptide linkage (ii) Primary structure (iii) Denaturation.
14.20
When two molecule of –amino acids similar or different combine in this way that the amino group of one molecule with carboxy group of the other molecule, this result in the elimination of water molecule and the formation of new bond-CO NH- .
This type of bond is called peptide bond or peptide linkage. The product of reaction is called dipeptide. For example, COOH group of valine combine with NH2 group of alanine, we get dipeptide Valylalanine (Val-Ala) as final product, as shown below:-
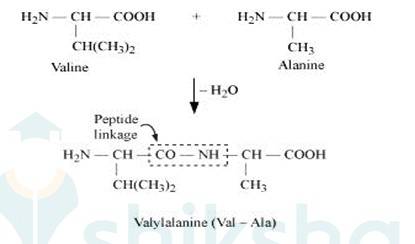
2- Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and due to this sequence of amino acids the structure of proteins is said to be primary structure.
Any change in this sequence of amino acids creates different proteins. In other words, the sequence in which various amino acids are arranged in a protein is called primary structure. The amino acid sequence of a protein determines its function and it’s critical of its biological activity.
3- Each protein in the biological system has a unique 3-D structure and has specific biological This is called native form of a protein. When a protein in its native form is subjected to physical changes such as change in temperature, pH, etc.
hydrogen bonds are broken. Due to cleavage of hydrogen bonds, unfolding of protein molecule occurs and the protein loses its biological activity. This loss of biological activity is called denaturation. During denaturation, 2- degree and 3-degree structures of proteins are destroyed but 1-degree structure remains intact.
As a result of denaturation, globular proteins are converted into fibrous proteins. In other words, denaturation leads to coagulation. That is why coagulated proteins are also called denaturated proteins.
14.16 What is the basic structural difference between starch and cellulose?
14.16
Starch consists of two components – amylase and amylopectin. Amylose is a long linear chain of α–D-(+)-glucose units joined by C1-C4glycosidic linkage (α -link).
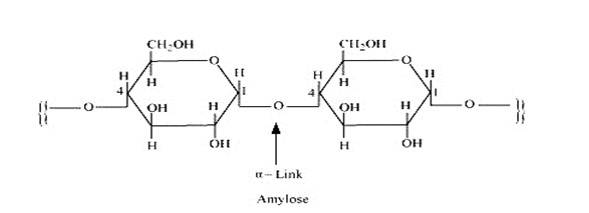
Amylopectin is a branched-chain polymer of –D-glucose units, in which the chain is formed by C1-C4 glycosidic linkage and the branching occurs by C1-C6 glycosidic linkage.

On the other hand, cellulose is the main structural material of tree and other plants. Wood is 50% cellulose, while cotton wool is almost pure cellulose. It is linear chain natural polymers of β-D-glucose units joined by 1, 4-glycosidic linkage (natural linear polymers).

14.9 What are monosaccharides?
14.9
Monosaccharides are the only the simplest units of carbohydrates and they are the simplest form of sugar. In other words, It is the most basic form of carbohydrates. They are made up of hydrogen, carbon and oxygen atoms. They are the building blocks of more complex carbohydrates i.e., they can join together and form complex carbohydrates.
For example:
i. Monosaccharides form
ii. 3-10 of them form
iii. 11 or more of them form
Monosaccharides are used to produce and store energy. Most organisms create energy by breaking down the monosaccharide glucose and harvesting the energy released from the bonds. Other Monosaccharides are used to form long fibers, which can be used as a form of cellular structure.
Examples of Monosaccharides are glucose, fructose, and galactose.

14.15 What are the hydrolysis products of (i) Sucrose and (ii) Lactose ?
14.15
Hydrolysis is the process of using water to break down a molecule into two parts. It is usually a type of decomposition reaction where one reactant is water, where water is used to break chemical bonds in the other reactant. It can be considered as reverse of a condensation reaction.
The general formula of a hydrolysis reaction is:
XY + H2O → XH + YOH
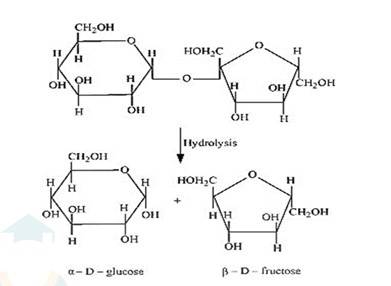
(i) On hydrolysis with dilute acids, sucrose yields an equimolecular mixture of α –D glucose and β–D- fructose.
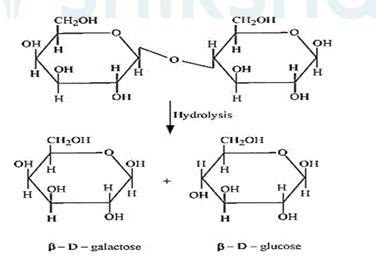
(ii) The hydrolysis of lactose gives β–D-galactose and β–D-glucose as final products.
14.25 What are enzymes?
14.25
Enzymes are naturally occurring simple conjugate proteins acting as specific catalysts in all processes. In contrast to ordinary chemical catalyst, it loses activity by pH or temperature change.
For example:- the enzyme used to catalyze the hydrolysis of maltose into glucose is named as maltase.

Enzymes are highly specific, i.e., a particular enzyme catalyses a specific reaction. For example, urase attacks on urea. This specific action is due to active sites present in the enzyme molecule (E) that fits into substrate (S) and forms E-S complex which changes into product P and E.
Enzymes increase the speed of reactions. They can catalyze several million of reactions per second.
14.14 What is glycogen? How is it different from starch?
14.14
Glycogen is a polysaccharide-type of carbohydrate. In animals, carbohydrates are stored as glycogen.
But starch is a carbohydrate which consists of two components –amylase (15 -20 %) and amylopectin (80 – 85%). However, glycogen is also like amylopectin but branching will take place after every 5 to 6 glucose unit. Also, glycogen is highly branched.
14.7 What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
14.7
When a nucleotide from DNA containing thymine is hydrolyzed β-D-2 deoxyribose and phosphoric acid are obtained.
14.5 Where does the water present in the egg go after boiling the egg?
14.5
Eggs contain protein and proteins are a polymer in amino acids. When an egg is boiled the protein present inside the egg is denatured (the process of loss of biological activity of protein like their ability of –H bonding when subjected to a physical change like a change in temperature, pH) and coagulated (the process of liquid changing to a solid or semi-solid state). Due to this, the water present in the egg is absorbed by the coagulated protein by –H bonding.
14.6 Why cannot vitamin C be stored in our body?
14.6
Vitamin C (ascorbic acid due to extensive –H bonding with water due to the presence of –OH group) is a water-soluble vitamin in contrast to vitamin A, D, E and K which are fat soluble. Also, humans cannot synthesize it due to the lack of specific enzyme and it is rapidly absorbed from the intestine.
Because it is water-soluble, it is not stored in our body to a significant amount but is readily excreted in the urine.
14.8 When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
14.8
A DNA is a double-stranded molecule (two polynucleotide chains whose nitrogenous bases are connected by hydrogen bonds).
In this molecule pairing or connection of bases occurs. Adenine always pairs with thymine, while cytosine always pairs with guanine.
So when DNA is hydrolyzed the amount of adenine produced is exactly equal to the amount of thymine produced, similarly, the amount of cytosine produced is equal to that of guanine.
But as given when RNA is hydrolyzed, there is no relationship between the quantities of different bases obtained. This suggests that RNA is a single-stranded molecule.
14.13 What do you understand by the term glycosidic linkage?
14.13
The condensation of the hydroxyl group of two monosaccharides to form a link between them is called glycosidic linkage. In other words, it refers to linkage developed between two different monosaccharide units through an oxygen atom by the loss of a water molecule. For example, in a sucrose molecule, two monosaccharide units, α-glucose and β–fructose, are joined together by a glycosidic linkage.
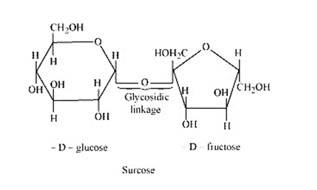

14.12 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
14.12
i. Ribose, 2-deoxyribose, galactose, and fructose are
Monosaccharides are the simplest units of carbohydrates which cannot be hydrolyzed into simpler compounds.
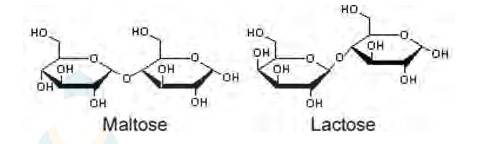
ii. Maltose and lactose are
A disaccharide is a carbohydrate that is formed when two monosaccharides are joined together and a molecule of water is removed from the structure. Lactose is a disaccharide formed from the combination of galactose and glucose.

14.22 What type of bonding helps in stabilising the α-helix structure of proteins?
14.22
The stability of α-helix structure is due to intramolecular hydrogen bonding between –NH and –CO groups of polypeptide chain. The α-helix is known as 3.613 because each turn of α-helix contains approximately 3.6 amino acids and a 13-membered ring is formed by hydrogen bonding.
14.26 What is the effect of denaturation on the structure of proteins?
14.26
During denaturation, 2-degree and 3-degree structures of proteins are destroyed but 1- degree structure remains intact. As a result of denaturation, The globular proteins (soluble in water) are converted into Fibrous proteins (insoluble in water) and their biological activity is lost. For example, boiled egg which contains coagulated proteins cannot be hatched to produce chickens.
14.33 What are the different types of RNA found in the cell?
14.33
The different types of RNA found in the cell are listed below:-
(i) Messenger RNA (m-RNA)
It carries the genetic message code from the DNA to ribosomes. It is produced by the DNA; m-RNA is also single stranded and constitutes about 15% of total RNA.
(ii) Ribosomal RNA (r-RNA)
It is found in the ribosomes and it is usually associated with protein to form the ribosomes. It is synthesised in the nucleus by DNA. It is single stranded, comprising about 80% of total RNA. It is metabolically stable.
(iii) Transfer RNA (t-RNA)
It is synthesised in nucleus by DNA. It is also called soluble RNA. It is single stranded. There are 20 different kinds of t-RNA and each type has specificity for a particular amino acid. It constitutes about 5% of total RNA. It has very short life.
14.4 The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
14.4
Amino acids are organic compounds containing amine (basic) and carboxyl (acidic) functional group with a specific side chain. Both acidic and basic group are present in the same molecule. In, aqueous solution carboxyl group can lose a proton (H+) and amino group can accept a proton (H+) giving rise to the dipolar ion called as zwitter ion. Zwitter ion is shown below:
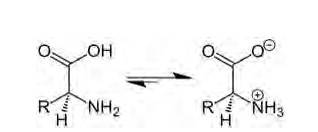
In this zwitter ion there is the presence of both positive as well as negative charge, so there is the development of strong electrostatic force of attraction between the molecules and the water. For this reason solubility of amino acids is higher. Due to strong electrostatic interaction there is formation of strong bond between them, hence the melting point of amino acid is higher than the halo acids (as they do not exhibit such dipolar behaviour).
14.11 Write two main functions of carbohydrates in plants.
14.11
Carbohydrates are an essential compound for the organic life It is used as primary source of energy by plants and animals. It also fulfill other needs like synthesizing of other chemicals and provide the structure for cells within the body.
Energy is stored in it in the form of starch which, provide either complex or simple type of sugars. Complex sugars i.e., polysaccharides, give a constant supply of energy while simpler sugars, like monosaccharides, supplies a quicker jolt before dissolving.
Animals receive these starches through foods, especially those made from plant life such as grains and bread.
Plants manufacture their own carbohydrates through photosynthesis, which uses energy absorbed from light to break up carbon dioxide and water into energy.
The two main functions of carbohydrates in plants are:-
i. Cellulose, a polysaccharide, is used to build the cell wall
ii. Polysaccharides such as starch serve as storage
14.17 What happens when D-glucose is treated with the following reagents? (i) HI (ii) Bromine water (iii) HNO3
14.17
When D-glucose is heated and treated with HI for a long period of time, then n-hexane is formed, which shows that all the six-carbon atoms are linked in a straight
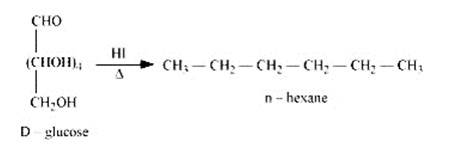
2. When D-glucose is treated with Br2 water i.e., bromine water which is a mild oxidising agent, then we get D-gluconic acid as one of the product. This reaction assures the presence of carbonyl group which is available as an aldehydic group.
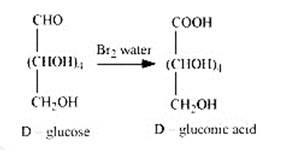
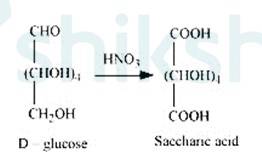
3. On being treated with HNO3, D-glucose get oxidised which gives saccharic acid as final Saccharic acid is a di-carboxylic acid. This reaction confirms the presence of alcoholic group (- OH) in the glucose.
14.19 What are essential and non-essential amino acids? Give two examples of each type.
14.19
(i) The amino acids which cannot be synthesised is called as essential amino acids. It must be obtained through diet. Example:- valine and leucine.
(ii) The amino acids which cannot be synthesised in the body is called as non-essential amino acids. Example: - glycine, Alanine.
14.21 What are the common types of secondary structure of proteins?
14.21
There are two common types of secondary structure of proteins:
(i) α–helix structure
(ii) β–pleated sheet structure
α–Helix structure
If the size of R-groups is quite large then the intramolecular bonds are formed between the C=O of one amino acid and the N-H group of the forth amino acid residue in the chain. This causes the polypeptide chain to coil up into a spiral structure called righ handed α-helix structure.
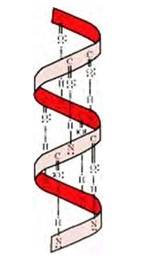
β -pleated sheet structure
In this conformation, the polypeptide chains lie side by side in a zig0zag manner with alternate R groups on the same side situated at fixed distances apart. The two such neighbouring polypeptide chains are held together by interbounded to form a sheet. These sheets are then stacked one above the other like the pages of the book to form a 3-D structure. This structure resembles pleated folds of drapery and hence is called β–pleated sheet structure. The polypeptide chains can link together in parallel and anti- parallel sequence. Such sheet like structure can easily slip on each other. Proteins of this structure are soft.
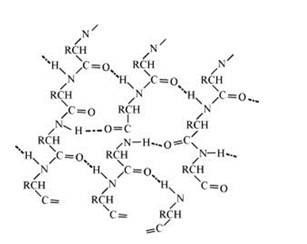
14.23 Differentiate between globular and fibrous proteins.
14.23
Globular proteins | Fibrous proteins |
It is usually soluble in water. | It is insoluble in water. |
All enzymes are globular protein. Some hormones such as insulin are also globular protein. | Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair; collagen in tendons; and myosin in muscles. |
The polypeptide chain in this protein is folded around itself, giving rise to spherical structure. | It is a fibre-like-structure formed by the polypeptide chain. These proteins are held together by strong and disulphide bonds. |
They are soluble in acids and bases. | They are insoluble in acids and bases. |
Examples:- egg albumin, casein of milk. | Examples :- Silk, Skin, Wool. |
They have weak intermolecular hydrogen bonding. | They have comparatively stronger intermolecular forces of attraction. |
They have folded, ball like structure. | They have thread like structure. |
Globular proteins are also called as spheroproteins owing to their shape. | Fibrous proteins are also called as scleroproteins. |
More sensitive to the changes in pH, temperature etc. | Less sensitive to the changes in pH, temperature etc. |
14.24 How do you explain the amphoteric behaviour of amino acids?
14.24
Amino acids contain an acidic (carboxyl group) and a basic (amino) group within the same molecule. In aqueous solution, they neutralize each other. The carboxyl group loses a proton while the amino group accepts it. S a result, a dipolar or zwitter-ion is formed.
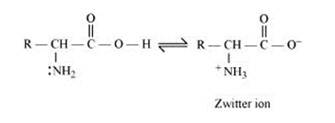
In zwitter ionic form, the amino acid show amphoteric behaviour as they react with both acids and bases. In the acidic medium, COO- ion of the zwitter-ion accepts a proton to form the cation first, while in the basic medium, +NH3 ion loses a proton to form the anion, as shown below:-
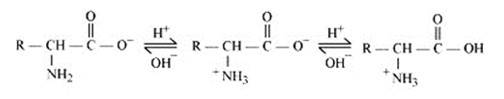
14.27 How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
14.27
Vitamins can be defined as the essential dietary factors required by an organism in minute quantities and whose absence causes specific deficiency Vitamins are broadly classified into two types:-
(i) Fat-soluble vitamins:
These are oily substances not readily soluble in water. These include vitamins A, D, E and K. Vitamin K is responsible for the coagulation of blood.
(ii) Water-soluble vitamins:-
Vitamins that are soluble in water belong to this group. For example :B group vitamins (B1, B2, B6, B12, etc.) and vitamin C.
Vitamin H (Biotin) as an exception, it is neither soluble in water nor in fat
14.28 Why are vitamin A and vitamin C essential to us? Give their important sources.
14.28
The deficiency of Vitamin A leads to Xerophthalmia which is hardening of the cornea of the eye and night blindness as well. Vitamin-C is essential to us because its deficiency causes scurvy which is a phenomenon of bleeding gums; and pyorrhoea which is phenomenon of loosening-bleeding of teeth.
The sources of vitamin A are fish, cod liver oil, carrots, butter and milk.
The sources of vitamin C are citrus fruits, lemon, amla and green leafy vegetables.
14.29 What are nucleic acids? Mention their two important functions.
14.29
Nucleic acids are Biomolecules which are found in the nuclei of all living cells, inform of nucleoproteins or chromosomes (proteins containing nucleic acids as the prosthetic group). Nucleic acids are of two types: – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are also known as Polynucleotide as they are long- chain polymers of nucleotides.
The two important functions of nucleic acids are listed below:-
(i) DNA which is responsible for the transference of hereditary effects from one generation to another, which is due to their property of replication during cell division as a result of which two identical DNA strands are transferred to the daughter
(ii) Nucleic acids (both DNA and RNA) are responsible for synthesis of all proteins needed for the growth and maintenance of our body. Actually, the proteins are synthesised by various RNA molecules in the cell but the message for the synthesis of a particular protein is given by DNA molecules
14.32 Write the important structural and functional differences between DNA and RNA.
14.32
The structural difference between DNA and RNA are as follows:-
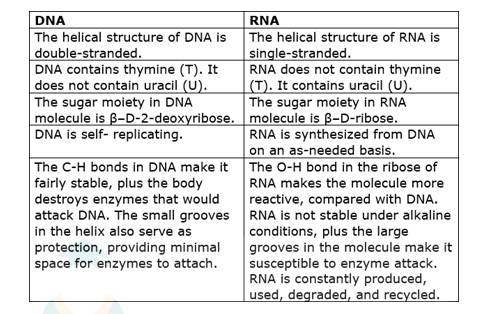
The functional difference between DNA and RNA are as follows:-

14.1 Glucose or sucrose is soluble in water but cyclohexane or benzene (simple six membered ring compounds) is insoluble in water. Explain.
14.1
Glucose and sucrose are carbohydrates (optically active polyhydroxy aldehydes or ketones).
Structure of glucose:
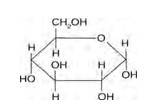
Structure of sucrose:
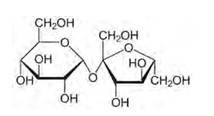
As you can see both the compounds have five –OH and eight –OH groups respectively. These –OH groups are responsible for the extensive hydrogen bonding with water. This –H bonding is responsible for the solubility of glucose and sucrose in water.
In case of cyclohexane or benzene (simple six-membered ring compounds), they do not contain any – OH groups. Hence, they cannot undergo –H bonding with water and are insoluble.
14.2 What are the expected products of hydrolysis of lactose?
14.2
Lactose is a disaccharide carbohydrate (made up of two monosaccharide units) composed of β-D-galactose and β-D-glucose units. Hydrolysis breaks the glycosidic bond converting sucrose into β-D- galactose and β-D-glucose.
NOTE: But however, this reaction is so slow that it takes years for the solution of sucrose to undergo negligible change. Hence an enzyme called sucrase is added to proceed rapidly.
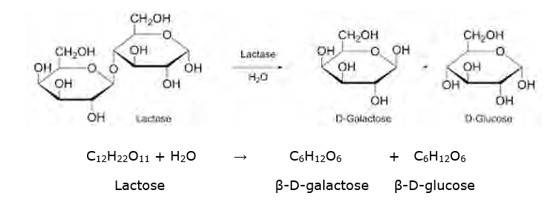
14.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
14.3
D-glucose reacts with hydroxylamine (NH2OH) to form oxime due to the presence of the aldehyde functional group (-CHO). This is due to the cyclic structure of glucose which forms an open chain structure in an aqueous medium, which then reacts to give an oxime.
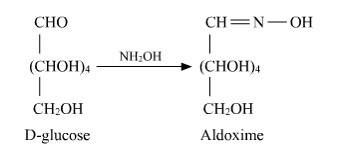
But in case of pentaacetate of D-glucose, it does not form open chain structure in an aqueous medium so it does not react with NH2OH.
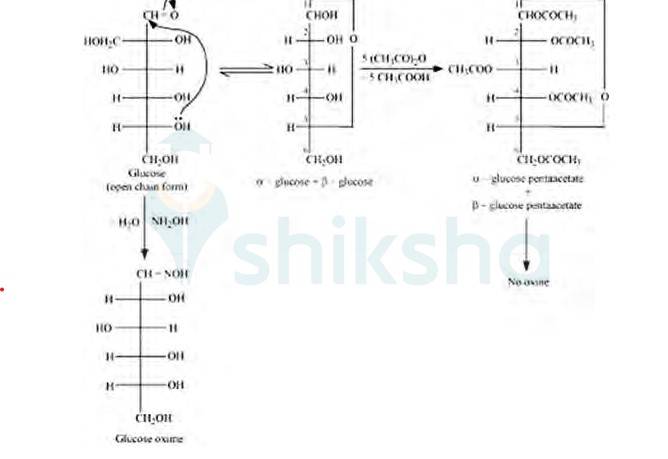
14.30 What is the difference between a nucleoside and a nucleotide?
14.30
A nucleoside is formed when l-position of a pyrimidine (cytosine, thymine or uracil) or 9- position of a purine (guanine or adenine) base is attached to C-l of sugar (ribose or deoxyribose) by a linkage. Thus in general, nucleosides may be represented as: Sugar-Base.
Nucleoside = sugar + base
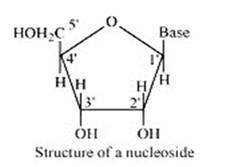
On the other hand, all the three basic components of nucleic acids (i.e., pentose sugar, phosphoric acid and base) are present in a nucleotide.These are obtained by esterification of C5' –OH group of the pentose sugar by phosphoric acid. Thus, in general, a nucleotide is represented as:-
Nucleotide= sugar + base + phosphoric acid
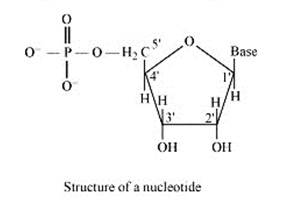
14.31 The two strands in DNA are not identical but are complementary. Explain.
14.31
In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases.
Cytosine from hydrogen bond with guanine, while adenine forms hydrogen bond with thymine. As a result, the two strands are complementary to each other.
DNA consists of two strands of nucleic acid chains coiled around each other in the form of a double helix. The base of one strand of DNA is paired with bases on other strand by means of hydrogen bonding.
This hydrogen bonding is very specific as the bases can only base pair in a complementary manner.
Adenine pairs with only thymine via 2 hydrogen bonds and guanine pairs with cytosine through 3 hydrogen bonds.
Thus, the two strands of DNA are complementary to each other in the sense that the sequence of bases in one strand automatically determines that of the other. So the DNA stands cannot be identical, but they are complementary to each other.
Benefit of using NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
- Familiarise yourself with various questions on a single topic
- Enhance Conceptual knowledge
- Improve problem-solving skills
- Help to improve time management skills
- Best for practice and revision
- Best resource to prepare for the board exam and other entrance tests
Chemistry Ncert Solutions Class 12th Exam
