Carbohydrates are a vast group of naturally occurring organic compounds. They are primarily produced by plants and form a significant portion of our food. The term "carbohydrate" literally means "hydrates of carbon," as their general formula was initially thought to be. However, this definition isn't entirely accurate, as some carbohydrates don't fit this formula, and some compounds with this formula are not carbohydrates.
Chemically, carbohydrates are defined as optically active polyhydroxy aldehydes or ketones, or compounds that produce such units upon hydrolysis. Many carbohydrates have a sweet taste and are commonly known as sugars.
This article teaches students about carbohydrates, their classification, types of carbohydrates, and more. We aim to guide Class 12 students in their exam preparation through this article. Moreover, we have uploaded the NCERT solutions for Class 11 and 12 online. Additionally, the expert teacher at Shiksha has prepared Class 12 Chemistry NCERT solutions for the textbook in-text and exercise questions.
- Carbohydrates Definitions
- Classification of Carbohydrates
- Reducing and Non-Reducing Sugars:
- Examples of Carbohydrates
Carbohydrates Definitions
The NCERT definition for Carbohydrates is, "Carbohydrates are primarily produced by plants and form a very large group of naturally occurring organic compounds. Some common examples of carbohydrates are cane sugar, glucose, starch, etc." The general formula of carbohydrates is Cn(H2O)n.
Explanation: Carbohydrates are organic compounds that occur in plants in large groups. Sugar cane, glucose and starch are common examples of carbohydrates.
Importance of Carbohydrates
Carbohydrates are important macronutrients that provide the energy for the body to function. The importance of carbohydrates is mentioned below.
- Essential for life in both plants and animals.
- Used as starch in plants and glycogen in animals
- Good for the proper functioning of the brain. A lack of carbohydrates will lead to poor concentration and mental fog.
- When carbohydrates are taken in enough quantity, proteins can be used for the development and maintenance of tissues.
- Help in maintaining blood sugar by consuming fibre-rich carbohydrates.
Classification of Carbohydrates
There are various types of carbohydrates, including monosaccharides, oligosaccharides, and polysaccharides. They are classified based on their behaviour when broken down (hydrolyzed).
Monosaccharides
- They are the simplest carbohydrates, and we can not split them into smaller units by hydrolysis.
- They are known as simple sugars.
Monosaccharides are classified in two ways:
- By number of carbon atoms:
- Triose (3 carbons)
- Tetrose (4 carbons)
- Pentose (5 carbon)
- Hexose (6 carbon)
- By functional group:
- Aldose: contains an aldehyde group (-CHO)
- Ketose: contains a ketone group (C=O)
Examples:
Glucose is a type of monosaccharide, which is also known as dextrose. It has 6 carbon atoms along with an aldehyde group. So it is called an aldohexose. Glucose is found in fruits and honey.
Structure of Monosaccharide
Monosaccharides are available in two forms:
- Open-chain form (Fischer projection)
- Cyclic form (Haworth projection)
Example of monosaccharides:
- Fructose
- Galactose
- Ribose and Deoxyribose
Monosaccharides exist in solution as an equilibrium between their open-chain and cyclic (ring) forms. The cyclic forms are more stable and are usually represented by Haworth projections (five or six-membered rings).
Oligosaccharides
Oligosaccharides are types of carbohydrates. When we hydrolyze them, they give two to ten monosaccharide units. Oligosaccharides are classified as:
- Disaccharides
- Trisaccharides
Glycosidic Linkage
- The bond between monosaccharides in disaccharides (and polysaccharides).
- It is formed by the reaction between the anomeric carbon of one monosaccharide and a hydroxyl group of another, with the elimination of a water molecule.
Important Disaccharides
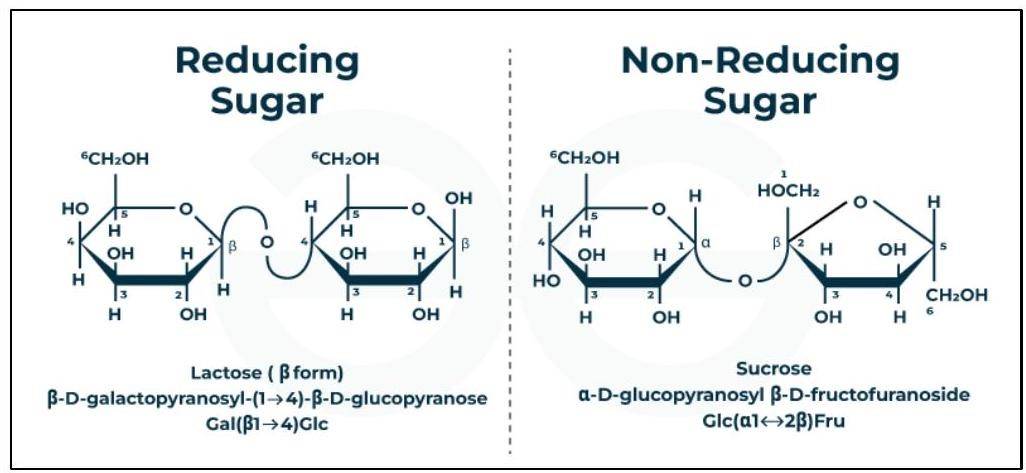
- Sucrose (table sugar): Composed of one molecule of glucose and one molecule of fructose. The glycosidic linkage is between C1 of -glucose and of -fructose. It is a non-reducing sugar.
- Lactose (milk sugar): Composed of one molecule of galactose and one molecule of glucose. The glycosidic linkage is between C1 of -galactose and C4 of glucose. It is a reducing sugar.
- Maltose (malt sugar): Composed of two molecules of a-glucose. The glycosidic linkage is between C1 of one glucose and C4 of the other. It is a reducing sugar.
Polysaccharides
These are complex carbohydrates that yield a large number of monosaccharide units upon hydrolysis. They are polymers of monosaccharides linked together by glycosidic linkages. Polysaccharides are often non-sweet and are known as non-sugars. They play important roles in energy storage and structural support.
Important Polysaccharides
- Starch: Carbohydrates are stored in plants as starch. It is a polymer of aglucose and consists of two components:
- Amylose: A linear polymer of a-glucose units linked by glycosidic bonds.
- Amylopectin: A branched polymer of a-glucose units with linkages in the main chain and linkages at the branch points.
- Cellulose: The main structural component of plant cell walls. It is a linear polymer of -glucose units linked by glycosidic bonds. Humans cannot digest cellulose due to the lack of the necessary enzymes.
- Glycogen: The storage polysaccharide in animals, found mainly in the liver and muscles. It has a structure similar to amylopectin but is more highly branched.
Reducing and Non-Reducing Sugars:
Carbohydrates can also be classified as reducing or non-reducing sugars based on their ability to reduce Fehling's solution and Tollens' reagent.
Reducing Sugar
These are the sugars that can reduce other substances like Fehling's solution or Benedict's solution. They have a free aldehyde or ketone group. The examples of reducing sugars are glucose, maltose, fructose, and lactose.
Non-Reducing Sugars
These sugar can not acts as reducing agents. The reactive groups of non-reducing sugars are tied up in a bond and are not free. Sucrose is a common example of a non-reducing disaccharide. The examples of non-reducing sugars are sucrose, gentianose, verbascose, raffinose, and trehalose.
Examples of Carbohydrates
Carbohydrates are organic compounds that serve as a source of energy. A few examples of carbohydrates are mentioned below
| Types of Carbohydrates | Sources |
| Simple Carbohydrates (Sugars) |
|
| Complex Carbohydrates (Starches) |
|
| Dietary Fiber |
|
Chemistry Biomolecules Exam
Student Forum
Other Topics under this Chapter
- Difference between Glucose and Fructose
- Hormones
- Enzymes
- Vitamins
- Proteins
- Carbohydrates
- Difference between Fat and Cholesterol
- Monomeric Proteins
- Difference between Starch and Cellulose
- Structure of Glucose and Fructose
- Functions of Nucleic Acids
- Glycosaminoglycans
- Uses of Ascorbic Acid
- Complex Carbohydrates
- Glycine structure
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test