Binding energy in Physics refers to the smallest amount of energy needed to remove a single particle from system of particles or for disassembling a system of particles into individual parts. In the next section, we will understand what is binding energy in more detail. Before moving further, please note that Binding energy related 1-2 questions are asked in the JEE Mains exam.
- What is Binding Energy?
- Types of Binding Energy
- Working Principle of Binding Energy
- Binding Energy Definition As Per NCERT
- Examples of Binding Energy
What is Binding Energy?
In simple words, binding energy definition is the minimum energy required for breaking the nucleus into constituent particles.
or
Amount of energy released during nucleus formation by its constituent particles and bringing them from the infinite separation.
Binding Energy (B.E.)
Note: Whenever the binding energy per nucleon is more for a nucleus then it is more stable.
For example
If
then nucleus 1 would be more stable.
Importance of Binding Energy
The following points highlight the importance of binding energy:
- Nuclei chapter is important for the CBSE Board exam and NEET exam as well.
- Binding energy is important in understanding how matter holds together at the most fundamental level. It explains why atoms are stable, measures the energy that is required to break apart nuclear particles, and governs nuclear reactions that fuel stars and nuclear reactors.
- Without binding energy, neither atoms nor molecules could exist, making it essential for all chemical and biological processes. It also determines which elements can undergo fusion or fission, making it central to nuclear physics and energy production.
Types of Binding Energy
The Nuclei chapter covers the topic of binding energy. It is advisable to practice the NCERT solutions of the Nuclei chapter. Let us now discuss the different types of Binding energy.
1. Gravitational binding energy
This type of binding energy is needed for expanding the material to infinity. It is the binding energy required for separating a gravitationally bound system into individual components.
2. Bond energy and bond-dissociation energy
These measure the binding energy amongst atoms within a chemical bond. This binding energy is used for disassembling molecule into constituent atoms. It seems similar to chemical energy release in chemical explosions and biological processes.
3. Ionization Energy
Ionization energy or electron binding energy refers to the measure of energy required for freeing an electron from a solid or its atomic orbital. This energy derives from electromagnetic interaction of electron with the nucleus as well as other electrons of an atom, solid or molecule.
Working Principle of Binding Energy
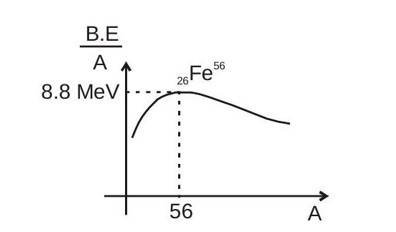
The binding energy per nucleon first increases on an average and reaches a maximum of about 8.8 MeV for . For still heavier nuclei, the binding energy per nucleon slowly decreases as increases. Binding energy per nucleon is maximum for , which is equal to 8.8 MeV . Binding energy per nucleon is more for medium nuclei than for heavy nuclei. Hence, medium nuclei are highly stable.
- The heavier nuclei being unstable have tendency to split into medium nuclei. This process is called Fission.
- The Lighter nuclei being unstable have tendency to fuse into a medium nucleus. This process is called Fusion.
Binding Energy Definition As Per NCERT
As per the NCERT, binding energy definition states that:
"Since the mass of the oxygen nucleus is less that the sum of the masses of its constituents (8 protons and 8 neutrons, in the unbound state), the equivalent energy of the oxygen nucleus is less than that of the sum of the equivalent energies of its constituents. If one wants to break the oxygen nucleus into 8 protons and 8 neutrons, "
This energy required Eb is related to the mass defect by
The above equation is the binding energy formula.
If a certain number of neutrons and protons are brought together to form a nucleus of a certain charge and mass, an energy Eb will be released in the process. The energy Eb is called the binding energy of the nucleus. We can think of binding energy per nucleon as the average energy per nucleon needed to separate a nucleus into its individual nucleons.
Examples of Binding Energy
Examples of Binding Energy are as follows:
1. Uranium-235 Fission
- U-235 nucleus splits into two fragments
- Typical reaction: U-235 + neutron → Ba-141 + Kr-92 + 3 neutrons
- Mass before: 235.0439 u + 1.0087 u = 236.0526 u
- Mass after: 140.9144 u + 91.9264 u + 3(1.0087 u) = 235.8671 u
- Mass defect: 236.0526 - 235.8671 = 0.1855 u
- Energy released: 0.1855 × 931.5 MeV = 172.8 MeV per fission
2. Hydrogen Fusion in Stars
- 4 hydrogen nuclei → 1 helium nucleus + 2 positrons + 2 neutrinos
- Mass before: 4 × 1.007825 u = 4.03130 u
- Mass after: 4.00260 u (helium) + 2 × 0.000549 u (positrons) = 4.00370 u
- Mass defect: 4.03130 - 4.00370 = 0.02760 u
- Energy released: 0.02760 × 931.5 MeV = 25.7 MeV per fusion
Physics Nuclei Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter