Radioactivity refers to a process where unstable atoms start to change. Since the nucleus is not balanced, it releases energy. This energy is known as radiation. This process goes on quietly in the background of nature such as in rocks and in the atmosphere. Scientists first noticed it by observing how materials like uranium changed. From there, they figured out that different emissions (alpha, beta, and gamma rays) reveal something about atomic structure. Today, it shows up in carbon dating, in medical imaging, and even in space exploration.
Do remember that this chapter is imporatant for JEE Mains examination and students must be well prepared to answer all the conceptual questions related to the chapter.
- What is Radioactivity?
- Types of Radioactive Particle
- Equations Related to Radioactivity
- Working Principle of Radioactivity
- Real Life Application of Radioactivity
What is Radioactivity?
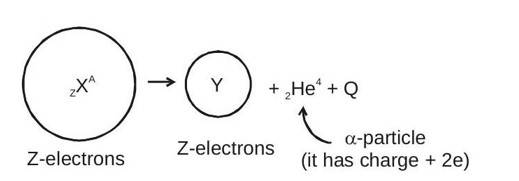
Radioactivity was discovered by Antoine Henri Becquerel. In simple terms, the spontaneous emission of radiations ( ) from unstable nucleus is called radioactivity. Substances that have radioactivity are known as radioactive substance. Radioactivity was studied in detail by Rutherford. In the radioactive decay, unstable nucleus emits particle or particle. After emission of or the remaining nucleus may emit - particle, and converts into more stable nucleus.
Please note: Radioactivity is an important concept covered in the nuclei chapter and it is important to practice NCERT solutions of radioactivity chapter.
Types of Radioactive Particle
1. -particle
This is a doubly charged helium nucleus that contains two protons and two neutrons each.
Mass of
-particle
Mass of
atom
Charge of
-particle
-particle :
(a)
(electron) :
Mass
; Charge
(b)
(positron) :
positron is an antiparticle of electron.
2. Antiparticle
A particle is called antiparticle of other if on collision both can annihilate (destroy completely) and converts into energy. For example : (i) electron (
) and positron (
) are anti particles. (ii) neutrino (v) and antineutrino (
) are antiparticles.
-particle : They are energetic photons of energy of the order of Mev and having rest mass zero.
Equations Related to Radioactivity
1. Radioactive Decay (Displacement Law) α-decay
Nuclei with mass number greater than 210 undergo -decay.
value : It is defined as energy released during the decay process.
Q value = rest mass energy of reactants - rest mass energy of products.
This energy is available in the form of increase in K.E. of the products.
Let,
mass of atom
mass of atom
mass of atom
.
value
Considering actual number of electrons in -decay
2. Calculation of Kinetic Energy of Final Products
As atom was initially at rest and no external forces are acting, so final momentum also has to be zero. Hence both and -particle will have same momentum in magnitude but in opposite direction.
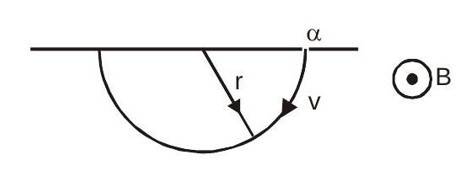
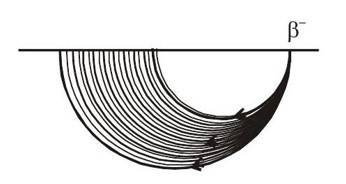
From the above calculation, one can see that all the -particles emitted should have same kinetic energy. Hence, if they are passed through a region of uniform magnetic field having direction perpendicular to velocity, they should move in a circle of same radius.
Working Principle of Radioactivity
Experimentally it has been observed that all the
-particles do not move in the circle of same radius, but they move in `circles having different radii.
This shows that they have different kinetic energies. But it is
also observed that they follow circular paths of some fixed
values of radius i.e. yet the energy of emitted
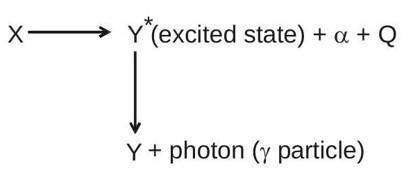
-particles is not same but it is quantized. The reason behind this is that all the daughter nuclei produced are not in their ground state but some of the daughter nuclei may be produced in their excited states and they emits photon to acquire their ground state.
The only difference between
and
is that
is in excited state and
is in ground state.
Let, the energy of emitted
-particles be E
- decay :
can also be written as
.
Here also one can see that by momentum and energy conservation, we will get
as
, we can consider that all the energy is taken away by the electron.
From the above results, we will find that all the
-particles emitted will have same energy and hence they have same radius if passed through a region of perpendicular magnetic field. But, experimental
observations were completely different.
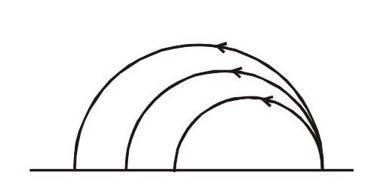
On passing through a region of uniform magnetic field perpendicular to the velocity, it was observed that
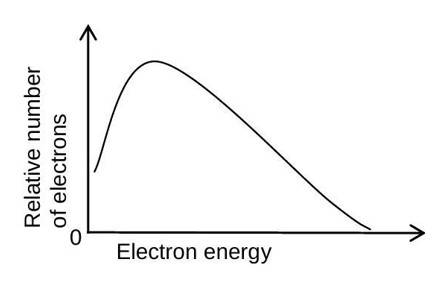
-particles take circular paths of different radius having a continuous spectrum.
To explain this, Paulling has introduced the extra particles called neutrino and antineutrino (antiparticle of neutrino).
Properties of antineutrino( & neutrino(v) :
(1) They have rest mass equal to zero or, at most, the mass equivalent of a few electronvolts.
speed = c (or nearly equal to c)
Energy,
(2) They are chargeless (neutral)
(3) They have spin quantum number,
Considering the emission of antineutrino, the equation of - decay can be written as
(4) They are not electromagnetic in nature as is the photon, the neutrino can pass unimpeded through vast amounts of matter.
Production of antineutrino along with the electron helps to explain the continuous spectrum because the energy is distributed randomly between electron and and it also helps to explain the spin quantum number balance ( and each has spin quantum number ).
During - decay, inside the nucleus a neutron is converted to a proton with emission of an electron and antineutrino.
Considering actual number of electrons.
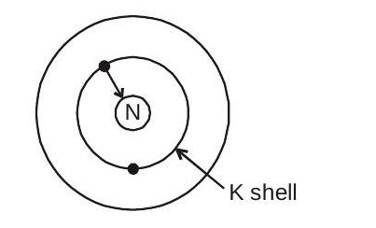
K-capture
It is a rare process which is found only in few nucleus. In this process the nucleus captures one of the atomic electrons from the K shell. A proton in the nucleus combines with this electron and converts itself into a neutron. A neutrino is also emitted in the process and is emitted from the nucleus.
Electron capture is competitive with positron emission. It occurs more often than positron emission in heavy nuclides because electrons are relatively closer to nucleus which allows more interaction.
If and are atoms then reaction is written as:
If and are taken as nucleus, then reaction is written as:
Real Life Application of Radioactivity
Like an atom a nucleus can also exist in states whose energies are higher than that of its ground state. Excited nuclei return to their ground states by emitting photons whose energies correspond to the energy differences between the various initial and final states in the transitions involved. The photons emitted by nuclei have energy up to several Mev, and are traditionally called gamma rays.
Al* represents aluminium nucleus in its excited state.
When -rays are passed through a slab their intensity decreases exponentially with slab thickness x .
where
is absorption coefficient. It depends on the slab.
Note : (1) Nuclei having atomic numbers from
to 112 shows radioactivity.
(2) Nuclei having to 83 are stable (only few exceptions are there)
(3) Whenever a neutron is produced, a neutrino is also produced.
(4) Whenever a neutron is converted into a proton, a antineutrino is produced.
The figure below shows the energy spectrum of the electrons emitted in the beta decay.
- decay :
In
decay, inside a nucleus a proton is converted into a neutron, positron and neutrino.
As mass increases during conversion of proton to a neutron, hence it requires energy for
decay to take place,
decay is rare process. It can take place in the nucleus where a proton can take energy from the nucleus itself.
value
Considering actual number of electrons.
value
Radioactivity is an important chapter from CBSE board exam and NEET exam point of view.
Physics Nuclei Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter