Nuclear energy is a carbon-free energy source that is formed due to nuclear energy. It happens when nuclei of an atom split into two or more parts, resulting in energy release. There are two ways of producing nuclear energy: through fission and fusion. We will be discussing both in later sections.
- What is Nuclear Energy?
- Types of Nuclear Energy
- Nuclear energy Equation
- Working Principle of Nuclear energy
- Practical Applications of Nuclear energy: Nuclear Reactor
- Fusion Reactions in Sun
What is Nuclear Energy?
Nuclear energy originates from the nucleus, the central part of an atom composed of protons and neutrons. This energy can be harnessed in two ways: through fission, where atomic nuclei split into smaller parts, or through fusion, where atomic nuclei combine. The amount of energy that can be derived is calculated through radioactive decay law.
Types of Nuclear Energy
Types of Nuclear Energy
The following are the different types of nuclear energy:
1. Nuclear Fission
This type of energy splits heavy atomic nuclei (like uranium-235 or plutonium-239) into lighter elements when struck by neutrons. It leads to the release of enormous amounts of energy. When a neutron collides with a heavy nucleus, it becomes unstable and splits into two smaller nuclei, which leads to the release of 2-3 neutrons and about 200 MeV of energy. This may trigger a number of fissions, which can ultimately lead to a chain reaction. Commercial nuclear power plants, nuclear-powered ships, submarines and weapons are all powered through nuclear fission. Nuclear fission has been a proven technology that has a very high energy density. It also does not lead to any greenhouse gas emissions during operations and has a reliable baseload power generation. While it has its own advantages, there are several disadvantages such as high construction cost, risk of accidents such as meltdowns, radioactive waste production with longer half-lives and proliferation risks.
2. Nuclear Fusion
It is also a type of nuclear energy that combines light atomic nuclei, such as hydrogen isotopes, under extreme conditions to form heavier elements. This results in the release of energy. For this to happen, nuclei must overcome strong electromagnetic repulsion at 100+ million degrees Celsius. The reaction is as follows:
deuterium + tritium → helium + neutron + 17.6 MeV
This type of nuclear energy has advantages since the fuel required, i.e. deuterium, is available in abundance from seawater. However, there are many technical challenges, such as high requirement of energy input requirements, expensive development costs and difficulty in commercialisation.
3. Radioactive Decay
This nuclear energy includes the spontaneous breakdown of the unstable atomic nuclei, which leads to the release of energy in the form of radiation and particles. This includes the alpha, gamma and beta decay. The process occurs whenever an unstable nuclei naturally transform into a more stable configuration, where each isotope has a characteristic half-life and releases energy during this transformation. Such a decay powers RTG for spacecraft, smoke detectors and emergency lighting systems. It is long-lasting, reliable power source that does not require any moving parts, has continuous energy output and works in extreme environments. There are certain disadvantages as well, such as low power output, radiation shielding requirements, gradual power decrease over time and limited availability of suitable isotopes.
4. Hybrid Fusion-Fission
In this type of nuclear energy, fusion reactions in the core are combined with fission reactions in the surrounding blanket. It is generated when fusion reactions produce high-energy neutrons. These neutrons are absorbed by a fissile or fertile blanket. This blanket material undergoes fission and multiplies the energy output. It has a lower fusion requirement as compared to pure fusion plants, efficient neutron use and can burn existing nuclear waste. While there are many advantages, there are certain disadvantages, such as safety concern related to both fusion and fission components and economic viability.
Please note: Nuclei chapter is important from the CBSE Board and NEET exam point as well.
Nuclear energy Equation
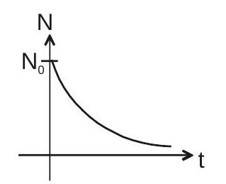
1. Radioactive Decay
Rate of radioactive decay
where
number of active nuclei
where
decay constant of the radioactive substance.
Decay constant is different for different radioactive substances, but it does not depend on amount of substance and time.
SI unit of
is
If
then first substance is more radioactive (less stable) than the second one.
For the case, if
decays to
with decay constant
Rate of radioactive decay of
(it is exponential decay)
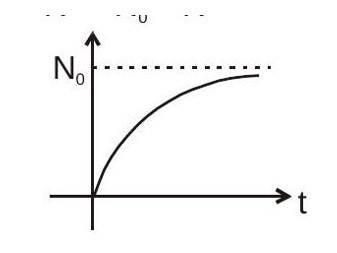
Number of nuclei decayed (i.e. the number of nuclei of
formed)
Working Principle of Nuclear energy
Nuclear energy is produced via nuclear fission and nuclear fusion in nuclear energy plant. To understand how is nuclear energy generated, the working principles of fission nuclear energy and nuclear energy fusion have been discussed below:
1. NUCLEAR FISSION
In nuclear energy fission, heavy nuclei of A, above 200, break up into two or more fragments of comparable masses. The most attractive bid, from a practical point of view, to achieve energy from nuclear fission is to use as the fission material. The technique is to hit a uranium sample by slow-moving neutrons (kinetic energy , also called thermal neutrons). A nucleus has large probability of absorbing a slow neutron and forming nucleus. This nucleus then fissions into two or more parts. A variety of combinations of the middle-weight nuclei may be formed due to the fission. For example, one may have
and a number of other combinations.
- On an average 2.5 neutrons are emitted in each fission event.
- Mass lost per reaction a.m.u.
- In nuclear fission the total B.E. increases and excess energy is released.
- In each fission event, about 200 MeV of energy is released a large part of which appears in the form of kinetic energies of the two fragments. Neutrons take away about 5 MeV .
eg. energy
value
- A very important and interesting feature of neutron-induced fission is the chain reaction.
2. NUCLEAR FUSION (THERMO NUCLEAR REACTION)
In nuclear energy fusion, some unstable light nuclei of A below 20, fuse together, the B.E. per nucleon increases and hence the excess energy is released. The easiest thermonuclear reaction that can be handled on earth is the fusion of two deuterons (
reaction) or fusion of a deuteron with a triton (
reaction) in the nuclear energy reactor.
value
M Нез
M нез
(D-D)
value
value
Note: In case of fission and fusion,
.
(b) These reactions take place at ultra high temperature ( to ). At high pressure it can take place at low temperature also. For these reactions to take place nuclei should be brought upto 1 fermi distance which requires very high kinetic energy.
(c) Energy released in fusion exceeds the energy liberated in the fission of heavy nuclei.
Practical Applications of Nuclear energy: Nuclear Reactor
Nuclear reactors utilize energy released in nuclear fission reaction to produce power. Some nuclear reactor are research reactors. Their primary aim is to provide a facility for research on different aspects of nuclear science and technology. Some reactors are used to produce power.
Important components of a nuclear reactor :
(i) Moderators : The average energy of neutrons liberated in fission of a
is 2 Mev . These neutrons unless slowed down will escape from the reactor without interacting with uranium nuclei. Fast neutrons need to be slowed down for them to be able to get absorbed by Uranium. When neutrons are made to strike a light nuclei like that of a hydrogen it looses almost all of it K. E. In reactor light nuclei called moderators are used. Commonly used moderators are water, heavy water (
) and graphite 'Apsara' reactor in BARC user
RAPP uses
as moderator
(ii) Multiplication factor (K) : The ratio of number of fissions produced by given generation of neutrons to the number of fissions of the preceding generation
If
, the operation of reactor is said to be critical. For steady generation of power
must be equal to 1
If
, the reaction rate and nuclear reactor power increases exponentially if
is not brought down the reactor will become super critical and may explode.
(iii) Control Rods :- The reaction rate is controlled through control-rods made out of neutron absorbing material such as cadmium.
(iv) Safety Rods : - These rods are provided in reactors in addition to control rods These, when required, can be inserted into the reactor and K can be reduced.
Fusion Reactions in Sun
The fusion reaction in sun is multi-step process which involves conversion of hydrogen in helium. The below set of reactions is called as cycle of nuclear fusion in stars
12.86 Mev
(ii) + (iii)
(iv)
Physics Nuclei Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter