
NCERT Solutions for Class 12 Chemistry Chapter 2 – Electrochemistry is a crucial part for the preparation of the CBSE Board examination and other entrance exams such as NEET and JEE Main. Electrochemistry NCERT solutions provide comprehensive and step-by-step explanations to all NCERT textbook questions. The solutions are designed to help the students strengthen their understanding of concepts and score high on the exams. It is ideal for revising concepts and solving complex problems. Electrochemistry Class 12 NCERT solutions cover the following key topics: electrochemical cells, conductance, Nernst equation, and electrolysis with clear diagrams and simplified answers.
For the topic-wise chemistry notes of Class 12, students must Explore Here. They will also get the solved examples.
- NCERT Class 12 Chemistry Chapter 2 Exercise Solutions - Key Topics, Weightage
- Electrochemistry Class 12 NCERT Solutions Important Formulas
- Class 12 Electrochemistry NCERT Solution PDF: Download PDF for Free
- Class 12 Chemistry Chapter 2 Electrochemistry NCERT Solutions
- Electrochemistry Class 12 Chemistry - FAQs
NCERT Class 12 Chemistry Chapter 2 Exercise Solutions - Key Topics, Weightage
Before one starts the chapter preparation, it is always a good idea to know what topics are covered in that chapter. Candidates can check here the list of all topics that are covered under the electrochemistry chapter of the NCERT chemistry class 12:
| Exercise | Topics Covered |
|---|---|
| 2.1 | Electrochemical Cells |
| 2.2 | Galvanic Cells |
| 2.3 | Nernst Equation |
| 2.4 | Conductance of Electrolytic Solutions |
| 2.5 | Electrolytic Cells and Electrolysis |
| 2.6 | Batteries |
| 2.7 | Fuel Cells & Corrosion |
Class 12 Electrochemistry Weightage in NEET, JEE Main
| Exam | Weightage |
|---|---|
| NEET | 3-4% |
| JEE Main | 3.3% |
For comprehensive preparation, refer to our Class 12 Chemistry NCERT Solutions with chapter-wise PDFs, key topics, and mark weightage to help you revise smartly and focus on exam-relevant content.
Electrochemistry Class 12 NCERT Solutions Important Formulas
The following are the important formulas of Class 12 Chemistry NCERT Solutions:
Important Formulae in Electrochemistry for CBSE and Competitive Exams
| Concept | Formula |
|---|---|
| Nernst Equation | |
| Faraday’s First Law | |
| Faraday’s Second Law | |
| Gibbs Free Energy | |
| Molar Conductance | |
| Kohlrausch’s Law |
Whether you're studying for CBSE board exams, JEE, or NEET, these revision notes can be your go-to resource for last-minute preparation. Strengthen your concepts and practice exam-relevant questions to boost your confidence and scores by exploring - Here.
Class 12 Electrochemistry NCERT Solution PDF: Download PDF for Free
Students must download the Electrochemistry NCERT PDF from the link given here. The solutions are well-structured, which improves the students' problem-solving skills. It is created by the Shiksha's subject matter experts. Students must download these accurate and reliable solutions for exam preparation.
Download Here: NCERT Solution for Class XII Chemistry Electrochemistry PDF
Looking for reliable resources to boost your exam prep? Access our NCERT Solutions for Class 11 and 12 Maths, Physics, and Chemistry. It provides chapter-wise free PDFs, covering all key topics as per the latest CBSE curriculum. Ideal for quick revision and concept clarity.
Class 12 Chemistry Chapter 2 Electrochemistry NCERT Solutions
Here we have provided Class 12 chemistry chapter 2 solutions for the Intext questions. Students can find the step by step solutions below.
| Q 3.1 How would you determine the standard electrode potential of the system Mg2+|Mg? |
| Ans To determine the standard electrode potential of the system Mg2+|Mg, connect it to the standard hydrogen electrode (SHE). Keep the Mg2+|Mg system as cathode and SHE as cathode. This is represented as shown below. |
| Q 3.2 Can you store copper sulphate solutions in a zinc pot? |
| Ans NO, because Zn is very reactive with Cu. It reacts with copper sulphate to form zinc sulphate i.e., Zn displaces Cu and metallic Cu is also formed. |
| Q 3.3 Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions. |
| Ans For a substance to oxidise Fe2+ to Fe3+ ion, it must have high reduction potential than Fe3+. The reduction potential of Fe3+ to Fe2+ reaction is 0.77V, the substances which have reduction potentials higher than this value will oxidise Fe2+ ions. Comparing the values, from the table: |
| Q 3.4 Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10. |
| Ans Given: ⇒ [H+] = 10 − 10 M |
Commonly asked questions
3.13 Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during recharging.
Anode: Lead (Pb)
Cathode: a grid of lead packed with lead oxide (PbO2)
Electrolyte: 38% solution of sulphuric acid (H2SO4)
The cell reactions are as follows :
Pb (s) + SO2-4 (aq) ⇒ PbSO4 (s) + 2e- (anode)
PbO2 (s) + SO2-4 (aq) + 4H+ (aq) +2e-⇒ PbSO4 (s) +2H2O (l) (cathode)
Pb (s) + PbO2 (s) +2H2SO4 (aq)⇒ 2PbSO4 (s) +2H2O (l)
(overall cell reaction)
On charging, all these reactions will be reversed.
3.20 Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s)|Mg 2+(0.001M)||Cu2+(0.0001 M)|Cu(s) (ii) Fe(s)|Fe 2+(0.001M)||H+ (1M)|H2 (g)(1bar)| Pt(s) (iii) Sn(s)|Sn2+(0.050 M)||H+ (0.020 M)|H2 (g) (1 bar)|Pt(s) (iv) Pt(s)|Br – (0.010 M)|Br2 (l )||H+ (0.030 M)| H2 (g) (1 bar)|Pt(s).
A 3.5 Ecell = ?
(i) Mg + Cu2+ → Mg2+ + Cu (n = 2)
E0 Cu2+ / Cu+ = 0.34V
E0 Mg 2+ / Mg = - 2.37 V
Ecell0 = ER0-EL0
Ecell0 = 0.34 - ( - 2.37) → Equation 1
Using Nernst equation, we get,
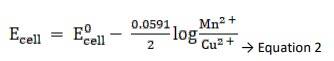
Substituting Equation 1 in equation 2, we get,
∴ Ecell = 0.34 - ( - 2.37) - 0.0591 / 2 log 10 -3/ 10-4
= 2.71 - 0.0591 / 2 log 10
= 2.71 - 0.02955
∴ Ecell = 2.68 V
The e.m.f of the cell, Ecell is 2.68 V
ii) Fe + 2H + → Fe2+ + H2 (n = 2)
E0 H+ / H2 = 0V
E0 Fe 2+ / Fe = - 0.44 V
Ecell0 = ER0-EL0
Ecell0 = 0 - ( - 0.44) → Equation 1
Using Nernst equation, we get,
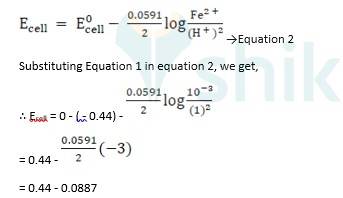
∴ Ecell = 0.5287 V
The e.m.f of the cell, Ecell is 0.5287 V
iii) Sn + 2H + → Sn2+ + H2 (n = 2)
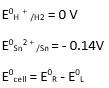
Ecell0 = 0 - ( - 0.14) → Equation 1
Using Nernst equation, we get,
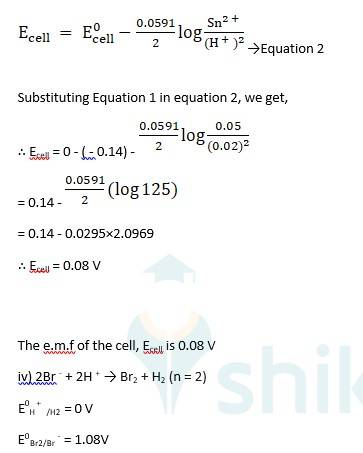
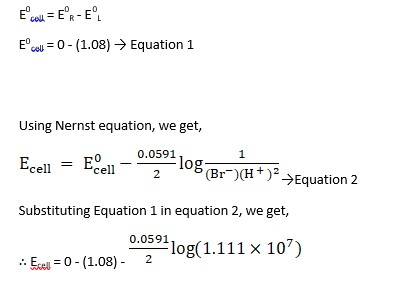
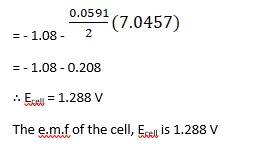
3.7 Why does the conductivity of a solution decrease with dilution?
The conductivity of a solution depends on the amount of ions present per volume of the solution. When diluted, the concentration of the ions decreases which implies that the number of ions per volume decreases thus, in turn, conductivity decreases.
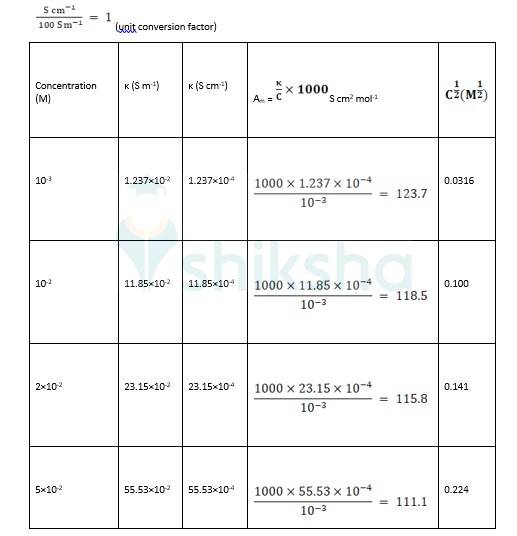
3.25 The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below: Concentration/M 0.001 0.010 0.020 0.050 0.100 102 × κ/S m^–1 1.237 11.85 23.15 55.53 106.74 Calculate Λm for all concentrations and draw a plot between Λm and c½ . Find the value of 0 Λm
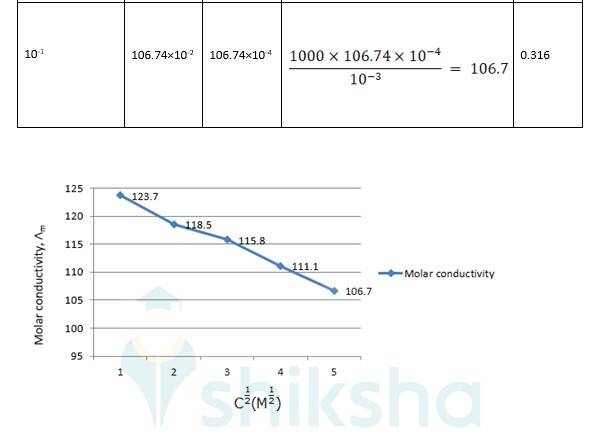
? 0 R = Intercept on the? axis = 124.0 S cm2 mol-1, which is obtained by extrapolation to zero concentration.
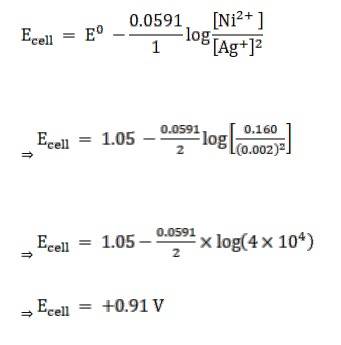
3.5 Calculate the emf of the cell in which the following reaction takes place: Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s) Given that E(cell) V = 1.05 V
Given:
[Ag+] = 0.002 M
[Ni2+] = 0.160 M
n = 2
(n = moles of e- from balanced redox reaction)
E0cell= 1.05 V
Now, using the Nernst equation, we get,
3.22 Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
The conductivity of a solution is defined as the conductance of one unit volume of solution kept between two platinum electrodes with a unit area of cross-section and at a distance of unit length.The molar conductivity of the solution is defined as the conducting power of all the ions produced by
one gram mole of an electrolyte in a solution. It is denoted by ∧m.
The conductivity of a solution (both for strong and weak electrolytes) always decreases with the decrease in concentration of the electrolyte i.e., on dilution. This pattern is seen because the number of ions per unit volume that carry the current in the solution decreases on dilution. The molar conductivity of the solution increases with the decrease in concentration of the electrolyte. This is because both the number of ions as well as mobility of ions increases with dilution.
3.4 Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Given:
For hydrogen electrode, pH = 10
n = 1
(n = moles of e- from balanced redox reaction)
On using the formula [H+] = 10– pH
⇒ [H+] = 10 − 10 M
We know,
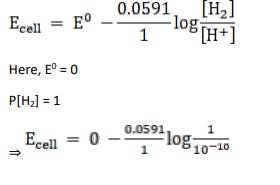
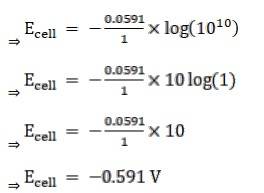
3.1 How would you determine the standard electrode potential of the system Mg2+|Mg?
To determine the standard electrode potential of the system Mg2+|Mg, connect it to the standard hydrogen electrode (SHE). Keep the Mg2+|Mg system as cathode and SHE as cathode. This is represented as shown below.
Pt (s) | H2 (g, 1 bar)| H+ (aq, 1 M) |Mg2+ (aq, 1M)| Mg
The electrode potential of a cell is given by
E? = E? R – E? L
Where,
E? R- Potential of the half-cell in the right side of the above representation
E? L- Potential of the half-cell in the left side of the above representation
It is to be noted that the potential of the standard hydrogen electrode is zero.
Therefore, E? L = 0
E? = E? R – 0
⇒ E? = E? R
3.33 Predict the products of electrolysis in each of the following: (i) An aqueous solution of AgNO3 with silver electrodes. (ii) An aqueous solution of AgNO3 with platinum electrodes. (iii) A dilute solution of H2SO4 with platinum electrodes. (iv) An aqueous solution of CuCl2 with platinum electrodes.
Given -
(i) All the ions are in aqueous state.
Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O → H + + OH -
At cathode:
Ag + (aq) + e - →Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference to H + ions.
At anode:
Ag (s)→ Ag + (aq) + e -
As Ag anode is attacked by NO3- ions, Ag of the anode will dissolve to form Ag + ions in the aqueous solution.
(ii) Reaction in solution:
AgNO3 (s) + aq → Ag + + NO3-
H2O óH + + OH -
At cathode:
2Ag + (aq) + 2e - →2Ag (s)
Ag + ions have lower discharge potential than H + ions. Hence, Ag + ions get deposited as Ag in preference to H + ions.
At anode:
2OH- (aq) → O2 (g) + 2H+ (aq) + 4e -
As anode is not attackable, out of OH - and NO3- ions, OH - having lower discharge potential, will be discharged in preference to NO3- ions. These then decompose to give out O2.
(iii) Reaction in solution
H2SO4 (aq) → 2H+ (aq) + SO2-4 (aq)
At cathode:
2H + (aq) + 2e - →H2 (g)
At anode:
2OH- (aq) → O2 (g) + 2H+ (aq) + 4e -
∴ H2 gas is evolved at cathode and O2 (g) is evolved at anode.
(iv) Reaction in solution: CuCl2 (s) + aq → Cu2+ (aq) + Cl- (aq)
H2O óH + + OH -
At cathode:
Cu2+ (aq) + 2e - →Cu (g)
At anode:
2Cl- (aq) - 2e- → Cl2 (g)
∴ Cu will be deposited at cathode and Cl2 gas will be liberated at anode.
3.19 Calculate the standard cell potentials of galvanic cell in which the following reactions take place: (i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd (ii) Fe 2+(aq) + Ag+ (aq) → Fe3+(aq) + Ag(s) Calculate the ?rG? and equilibrium constant of the reactions.
(1) Known - E0Cr3+/Cr = - 0.74 V
E0 cd2+ = - 0.40 V
? rG0 =? K =?
The galvanic cell of the given reaction is written as - Cr (s)|Cr3+ (aq)| Cd2+ (aq)|Cd (s)→ Reaction 1
Hence, the standard cell potential is given as, E0 = ER0 - EL0
= - 0.40 - (- 0.74)
∴ E0 = + 0.34 V
To calculate the standard Gibb’s free energy? rG0, we use,
? rG0 = - nE0F → Equation 1
wherenF is the amount of charge passed and E0 is the standard reduction electrode potential. Substituting n = 6 (no. of e - involved in the reaction 1), F = 96487 C mol-1,
E0 = + 0.34 V in Equation 1, we get, l
? rG0 = - 6×0.34V×96487 C mol-1
= - 196833.48 CV mol-1
= - 196833.48 J mol-1
∴? rG0 = - 196.83348 kJ mol-1
To find out the equilibrium constant, K, we use the formula,
log K =n E0 / 0.0591
=6 X 0.3 V / 0.0591
log K = 34.5177
K = antilog 34.5177
∴ K = 3.294 × 1034
The standard Gibb’s free energy? rG0 is - 196.83348 kJ mol –1 and equilibrium constant, K is 3.294 × 1034
(2) Known -
E0 Fe3+ / Fe2+ = 0.77V
E0 Ag+ / Ag = 0.80 V
? rG0 =? K =?
The galvanic cell of the given reaction is written as - Fe2+ (aq)|Fe3+ (aq)| Ag + (aq)|Ag (s)→ Reaction 1
Hence, the standard cell potential is given as, E0 = ER0 - EL0
= 0.80 - (0.77)
∴ E0 = + 0.03 V
To calculate the standard Gibb’s free energy? rG0, we use,
? rG0 = - nE0F → Equation 1
wherenF is the amount of charge passed and E0 is the standard reduction electrode potential.
Substituting n = 1 (no. of e - involved in the reaction 1), F = 96487 C mol-1, E0 = + 0.03V in Equation 1, we get,
? rG0 = - 1×0.03V×96487 C mol-1
= - 2894.61 CV mol -1
= - 2894.61 J mol-1
∴? rG0 = - 2.89461 kJ mol-1
To find out the equilibrium constant, K, we use the formula,
log K =n E0 / 0.0591
=1 X 0.03 V / 0.0591
log K = 0.5076
K = Antilog 0.5076
∴ K = 3.218
The standard Gibb’s free energy? rG0 is - 2.89461 kJ mol –1 and equilibrium constant, K is 3.218
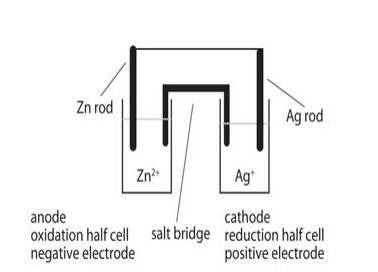
3.18 Depict the galvanic cell in which the reaction Zn(s)+2Ag+ (aq) →Zn2+(aq)+2Ag(s) takes place. Further show: (i) Which of the electrode is negatively charged? (ii) The carriers of the current in the cell. (iii) Individual reaction at each electrode.
The galvanic cell corresponding to the given redox reaction can be represented as:
Zn|Zn2+ (aq)|Ag + (aq)|Ag
- 1) Zn electrode (anode) is negatively charged because, at this electrode, Zn is oxidized to Zn2+, causing electron accumulation at the
- 2) Electrons (ions) are the carriers of the current in the cell and in the external circuit, current flows from Ag (cathode) to Zn (anode) which is normally opposite to the electron flow which is from anode to cathode.
- 3) At anode:
Zn (s)⇒ Zn2 + (aq) + 2e– At cathode:
Ag + (aq) + e –⇒ Ag (s)
3.16 Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn.
The order in which the given metals displace each other from the solution of their salts is given by,
Mg>Al> Zn> Fe> Cu
A metal of stronger reducing power displaces another metal of weaker reducing power from its solution of salt. The order of increasing the reducing power of given metals is Cu< Fe< Zn< Algiven metals displace each other from the solution of their salts is given by, Mg>Al> Zn> Fe> Cu. This is hence arranged in decreasing order of its reactivity
3.29 How much electricity is required in coulomb for the oxidation of (i) 1 mol of H2O to O2 ? (ii) 1 mol of FeO to Fe2 O3 ?
(i) The electrode reaction for 1 mole of H2O is given as,
H2O → H2 + 1/2O2
i.e., O2- →1/2 O2 + 2e -
∴ The quantity of electricity required = 2F
= 2×96487 C
= 192974 C
The quantity of electricity required in coulomb for the oxidation of 1 mol of H2O to O2 is 192974 C
(ii) The electrode reaction for 1 mole of FeO is
FeO + 1/2 O2 → 1/2 Fe2O3
i.e., Fe2+ → Fe3+ + e -
∴ The quantity of electricity required = 1F
= 1×96487 C
= 96487 C
The quantity of electricity required in coulomb for the oxidation of 1 mol of FeO to Fe2O3 is 96487 C
3.6 The cell in which the following reaction occurs: ( ) ( ) ( ) ( ) 3 2 2Fe 2I 2Fe I aq aqaq 2 s + − + + → + has 0 Ecell = 0.236 V at 298 K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction
Given:
2Fe3+ (aq) + 2I- (aq) → 2Fe2+ (aq) + I2 (s)
E0cell = 0.236V
n = moles of e- from balanced redox reaction = 2
F = Faraday's constant = 96,485 C/mol
T = 298 K.
Using the formula, we get
? rG0 = – nFE0cell
⇒? rG0 = – 2 × FE0cell
⇒? rG0 = −2 × 96485 C mol-1 × 0.236 V
⇒? rG0 = −45540 J mol-1
⇒? rG0 = −45.54 kJ mol-1
Now,
? rG0 = −2.303RT log Kc
Where, K is the equilibrium constant of the reaction
R is the gas constant; R = 8.314 J-mol-C-1
⇒ −45540 J mol-1 = –2.303× (8.314 J-mol-C-1)× (298 K) × (log Kc)
Solving for Kc we get,
⇒ logKc = 7.98
Taking antilog both side, we get
⇒ Kc = Antilog (7.98)
⇒ Kc = 9.6 × 107
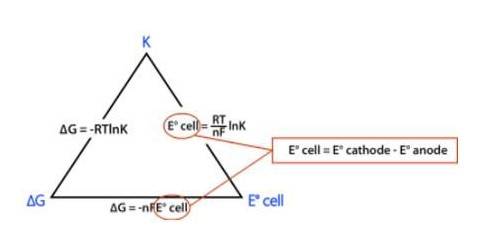
Trick to remember
3.21 In the button cells widely used in watches and other devices the following reaction takes place: Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH– (aq) Determine ?r G ? and E ? for the reaction.
Given - Zn → Zn2+ + 2e -, E0 = 0.76V (anode)
Ag2O + H2O + 2e - →2Ag + 2OH -, E0 = 0.344V (cathode), n = 2
ΔrG0 =?
Ecell0=?
Zn is oxidized and Ag2O is reduced.
Hence, the standard cell potential, Ecell0 is given as,
Ecell0 = ER0 - EL0
E0 cell = 0.344 + 0.76
∴ E0cell = 1.104 V
To calculate the standard Gibb’s free energy? rG0, we use,
? rG0 = - nE0F → Equation 1
= - 2×96487×1.104 J
= - 213043.296 J
∴? rG0 = - 2.13×105 J
The standard cell potential, E0cell is 1.104 V and the standard Gibb’s free energy? rG0 is - 2.13×105 J
3.15 Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
In the corrosion reaction, due to the presence of air and moisture, oxidation takes place at a particular point of an object made of iron. That spot behaves as the anode. The reaction at the anode is given by,
Fe (s) ⇒ Fe2+ (aq) + 2e-
Electrons released at the anodic spot move through the metal and go to another spot of the object, wherein presence of H+ ions, the electrons reduce oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from the air into water. The cathodic reaction is given by
O2 (air) + 4Haq++4e-⇒ 2H O
The overall reaction is given by,
2Fe (s) + O2 (air) + 4H (aq)+⇒ 2Fe2++2H2O
3.2 Can you store copper sulphate solutions in a zinc pot?
NO, because Zn is very reactive with Cu. It reacts with copper sulphate to form zinc sulphate i.e., Zn displaces Cu and metallic Cu is also formed.
The reaction is given as:
Zn + CuSO4 ⇒ ZnSO4 + Cu
3.24 The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 ?. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10^–3 S cm^–1 .
Given -
Resistance of a conductivity cell, R = 1500 Ω
Electrolytic conductivity of a solution, κ = 0.146 × 10-3 S cm-1
Cell constant =?
Conductivity, κ = cell constant/resistance
Cell constant = κ × R
= 0.146 × 10-3 S cm-1×1500 Ω
Cell constant = 0.219 cm-1
The cell constant of the cell containing 0.001M KCl solution at 298 K is 0.219 cm-1
3.8 Suggest a way to determine the Λm ° value of water
Kindly go through the solution

3.28 How much electricity in terms of Faraday is required to produce (i) 20.0 g of Ca from molten CaCl2?
(i) Ca2+ + 2e- → Ca
⇒ Here, 1 mole of Ca, i.e., 40g of Ca requires = 2 F electricity (F if Faraday)
∴ 20g of Ca requires = 20X2/40
= 1 F of electricity
Electricity in terms of Faraday required to produce 20.0 g of Ca from molten CaCl2 is 1 F of electricity.
(ii) Al3+ + 3e- → Al
⇒ 1 mole of Al, i.e., 27g of Al requires = 3 F electricity (F if Faraday)
∴ 40.0 g of Al will require = 3/27 X 40
= 4.44 F of electricity
Electricity in terms of Faraday required to produce 40.0 g of Al from molten Al2O3 is 4.44 F of electricit
3.17 Given the standard electrode potentials, (given below) Arrange these metals in their increasing order of reducing power.
K + /K = –2.93V, Ag+ /Ag = 0.80V, Hg2+/Hg = 0.79V Mg2+/Mg = –2.37 V, Cr3+/Cr = – 0.74V
A 3.2 Reducing power of metals increase with the decrease of reduction potential. Hence, the increasing order of reducing power will be as,
Ag < Hg < Cr < Mg < K
When the reduction potential is lower, the element has more tendency to get oxidized and thus more will be reducing power. The metal that has more negative electrode potential will be the one with more reducing power. Thus, here potassium (K) has the highest reducing power among the given elements.
3.14 Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
3.10 If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Current I = 0.5A
Time t = 2hrs = 2*60*60 = 7200 seconds
Charge Q = I * t
Q = 0.5*7200 = 3600 C
Charge carried by 1 mole of electrons (6.023*1023electrons) is equal to 96487C.
No of electrons = 6.023*1023 * 3600/96487
No of electrons = 2.25*1022 electrons
3.31 Three electrolytic cells A, B, C containing solutions of ZnSO4 , AgNO3 and CuSO4 , respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Equivalent weight is Ag, EAg = 180/1 = 180
Equivalent weight is Cu, ECu = 63.5 / 2 = 31.75
Equivalent weight is Zn, EZn= 65/2 = 32.5
Using Faraday’s second law of electrolysis, to find the mass of Cu and Zn, we use Equation 1,
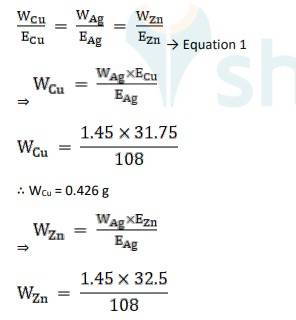
∴ WZn = 0.436 g
To find the time of current flow, using Faraday’s first law of electrolysis we get,
M = Z ×I ×t ⇒ Equation 2
? Z = Equivalent Weight / 96487, Equation 2 becomes,
M = 108 / 96487 X 1.5 X t
t = 1.45 X 96487 / 108X 1.5
t = 864 seconds.
The time of current flow, t = 864 seconds, the mass of Cu is 0.426 g and mass of Zn is 0.436 g
3.11 Suggest a list of metals that are extracted electrolytically.
Metals with greater reactivity can be extracted electrolytically. Sodium, potassium, calcium, lithium, magnesium, aluminium which are present in the top of the reactivity series are extracted electrolytically.
3.27 How much charge is required for the following reductions: (i) 1 mol of Al3+ to Al? (ii) 1 mol of Cu2+ to Cu? (iii) 1 mol of MnO4 – to Mn2+ ?
The electrode reaction is given as,
Al3+ (aq) + 3e- → Al (s)
∴ The quantity of charge required for the reduction of 1 mol of Al3+ = 3F
= 3×96487 C
= 289461 C
The electrode reaction is given as,
Cu2+ (aq) + 2e- → Cu (s)
∴ The quantity of charge required for the reduction of 1 mol of Cu2+ = 2F
= 2×96487 C
= 192974 C
The electrode reaction is given as, MnO4→ Mn2+
i.e., Mn7+ + 5e - → Mn2+
∴ The quantity of charge required for the reduction of 1 mol of Mn7+ = 5F
= 5×96487 C
= 482435 C
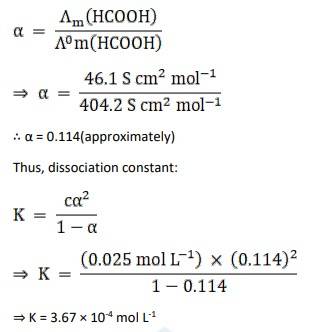
3.9 The molar conductivity of 0.025 mol L^–1 methanoic acid is 46.1 S cm^2 mol^–1 . Calculate its degree of dissociation and dissociation constant. Given λ0 (H+ ) = 349.6 S cm2 mol^–1 and λ 0 (HCOO– ) = 54.6 S cm^2 mol^–1 . s
C = 0.025 mol L-1
Am = 46.1 Scm2 mol L-1
λ0 (H+) = 349.6 Scm2 mol L-1
λ0 (HCOO-) = 54.6 Scm2 mol L-1
Λ0m (HCOOH) = Λ0 (H+) + Λ0 (HCOO-)
= 349.6 + 54.6
= 404.2 S cm2 mol L-1
Now, the degree of dissociation:
3.30 A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Quantity of electricity passed = 5 A × (20 × 60 sec)
= 6000 C ⇒ Equation 1
The electrode reaction is written as,
Ni2+ + 2e → Ni
Thus, the quantity of electricity required = 2F
= 2×96487 C
= 192974 C
? 192974 C of electricity deposits 1 mole of Ni, which is 58.7 g ⇒ Equation 2
Thus, equating equations 1 and 2, we get
192974 C of electricity deposits = 58.7 g
6000 C of electricity will deposit = 58.7 X 6000 / 192974
= 1.825g of Ni
The mass of Ni deposited at the cathode is 1.825g of Ni
3.23 The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm^–1. Calculate its molar conductivity
Given -
Molarity, C = 0.20 M
Electrolytic conductivity of a solution, κ = 0.0248 S cm-1
Molar conductivity =?
Molar conductivity, ∧m = K/C X 1000 S cm2 mol-1
=0.0248S cm-1 X 1000 Cm3L-1 / 0.20 mol L-1
∴ ∧m = 124 S cm2 mol-1
Molar conductivity (∧m) of 0.20 M solution of KCl at 298 K is 124 S cm2 mol-1
3.26 Conductivity of 0.00241 M acetic acid is 7.896 × 10–5 S cm–1. Calculate its molar conductivity. If 0 Λm for acetic acid is 390.5 S cm2 mol –1, what is its dissociation constant?
Given -
Molarity, C = 0.00241 M
Conductivity, κ = 7.896 × 10–5 S cm–1
Molar conductivity? m =?
? 0m for acetic acid = 390.5 S cm2mol–1
Molar conductivity? m = k/c X 1000 S cm2 mol-1
= 7.896 X 10–5 S cm–1X 1000 cm3 L-1 / 0.00241 mol L-1
∴? m = 32.76 S cm2 mol-1
To calculate the dissociation constant, Ka, we use
Ka = → Equation 1
Here, we need to find the value of α (degree of dissociation), by the formula,
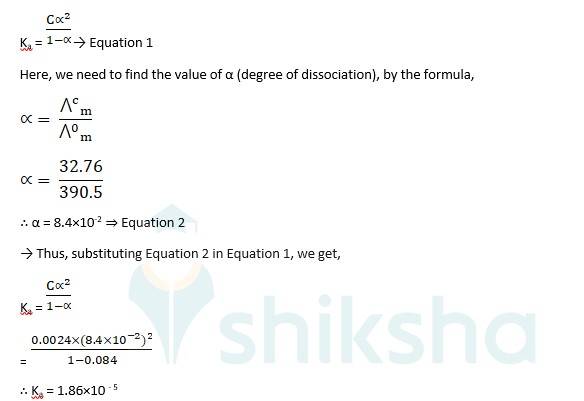
The molar conductivity? m is 32.76 S cm2 mol-1 and the dissociation constant, Ka is 1.86×10-5
3.3 Consult the table of standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
For a substance to oxidise Fe2+ to Fe3+ ion, it must have high reduction potential than Fe3+. The reduction potential of Fe3+ to Fe2+ reaction is 0.77V, the substances which have reduction potentials higher than this value will oxidise Fe2+ ions. Comparing the values, from the table:

3.12 Consider the below reaction. What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O7 2–?
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
A 3.12
Cr2O72– + 14H+ + 6e–⇒ 2Cr3+ + 7H2O
For reducing one mole of Cr2O72–, 6 mole of electrons are required. Hence, 6 Faraday charges is needed. Hence, 6F = 6×96487 = 578922 C. Thus, the quantity of electricity is needed is 578922 C.
3.32 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible: (i) Fe 3+(aq) and I – (aq) (ii) Ag+ (aq) and Cu(s) (iii) Fe3+ (aq) and Br– (aq) (iv) Ag(s) and Fe 3+ (aq) (v) Br 2 (aq) and Fe2+ (aq).
The electrode reaction is written as,
2Fe3+ + 2I - → 2Fe2+ + I2
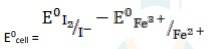
= 0.54V - 0.77V
∴ E0cell = - 0.23 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- The electrode reaction is written as,
- 2Ag+ (aq) + Cu(s)→ Cu2+ (aq) + Ag(s)
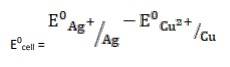
= + 0.80V - 0.34V
∴ E0cell = 0.46V
It is feasible, as Ecell 0 is positive, ∴ ?G0 is negative.
- (iii) The electrode reaction is written as,
- 2Fe3+ (aq) + 2Br- (aq)→ 2Fe2+ (aq) + Br2
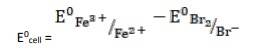 k
k= 0.77V - 1.09V
∴ E0cell = - 0.32 V
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- (iv) The electrode reaction is written as,
- Ag(s) + Fe3+ (aq) → Fe2+ (aq) + Ag+ (aq)
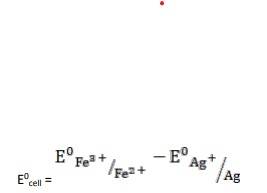
= 0.77V - 0.80V
∴ E0cell = - 0.03
It is not feasible, as E0cell is negative, ∴ ?G0 is positive.
- (v) The electrode reaction is written as,
- Br2 + 2Fe2+ (aq) → 2Br- (aq) + 2Fe3+ (aq)
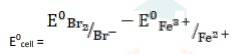
= 1.09V - 0.77V
∴ E0cell = 0.32 V
It is feasible, as Ecell0 is positive, ∴ ?G0 is negative.
Electrochemistry Class 12 Chemistry - FAQs
The following are the frequently asked questions from Class 12 Chemistry Chapter 2 Electrochemistry:
Commonly asked questions
What is electrochemistry? Describe according to Class 12 Chemistry.
In simple words, the study of relationship between electrical energy and chemical reactions is called the Electrochemistry. The concept comprises how chemical reactions can create electrical energy and how electrical energy can generate chemical changes.
Which is the basic principle of electrochemistry?
Redox reactions is the basic principle of the electrochemistry. The redox reactions is the process where electrons are transferred between substances. In this process chemical energy gets converted into electrical energy and vice versa.
Which are the types of electrochemical cells?
There are two types of electrochemical cells - Electrolytic and Galvanic or Voltaic cells. The electrolytic cells need an external source such as AC power source or DC battery and it involve non-spontaneous reactions. The galvanic cells gets its energy from redox reactions which is spontaneous.
Electrochemistry class 12 is hard or easy for students?
It depends on students. Though it is not a tough chapter to study but for students who have misconceptions and those who struggle with visualization can find it challenging.
Which are the real-world applications of electrochemistry?
The following are the real-world applications of electrochemistry - military applications such as thermal batteries, digital watches, hearing aids, digital cameras, electrical appliances such as cellphones, and torches.
Explore exams which ask questions on Chemistry Ncert Solutions Class 12th
Select your preferred stream
Chemistry Ncert Solutions Class 12th Exam
