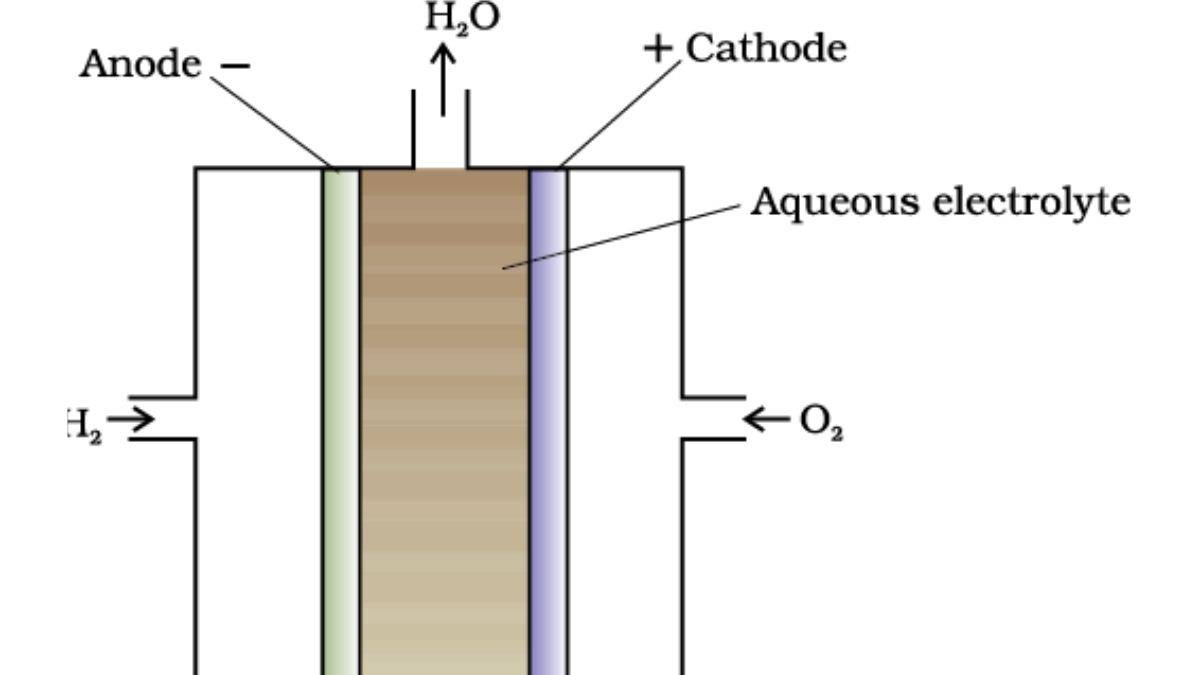
A fuel cell is a type of galvanic cell that can convert chemical energy into electrical energy through the redox reaction. This cell requires a continuous flow of fuel (hydrogen) and oxidant (oxygen) to generate electricity. Fuel cell is used in spacecraft, vehicles, and portable devices. The hydrogen-oxygen fuel cell is a key example.
Fuel Cell is an important topic in electrochemistry, along with batteries and electrochemical cells. Students can refer to the CBSE Class 12 Chemistry NCERT book for better understanding. The NCERT Chemistry book is the first preference of the topper when it comes to preparation. We have aslo provided short notes, a crisp version on fuel cells in this article.
Students can refer to the Class 12 Chemistry Chapter 2 Electrochemistry NCERT solutions to prepare for the CBSE board exam. The NCERT Solution is the best study material as it comprises many questions based on the topic. Previous year questions are focused on while preparing the Class 12 Chemistry study material. Moreover, an entrance exam like the JEE Main syllabus comprises electrochemistry topics.
Study Materials: Class 12 Chemistry NCERT Solution | NCERT Solution Class 12 Maths | Physics Class 12 NCERT Solutions
- What is Fuel Cell?
- Components of Fuel Cells
- Working of Fuel Cells
- Role of Porous Electrodes
- Types of Fuel Cells
- Thermodynamic Efficiency
- Advantage of Fuel Cells
- Cell Potential and Nernst Equation
- Applications of Fuel Cells
- Illustration for JEE Main
What is Fuel Cell?
The definition of a fuel cell as per the NCERT Class 12 Chemistry book is: Galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc., directly into electrical energy are called fuel cells.
Diagram of Fuel Cell
Note: Hydrogen and Oxygen are used to produce electricity.
Components of Fuel Cells
Fuel cells consist of:
- Anode: At the anode, oxidation takes place and electrons are released.
- Cathode: At the cathode, reduction occurs and consumes electrons.
- Electrolytes: An aqueous solution that contains free ions.
- Porous Electrodes: A type of electrode used in electrochemical devices. It allows gas diffusion and catalysis.
- External Circuit: This circuit connects electrodes for electron flow.
Aslo Read:
| NCERT Class 12 notes | |
| NCERT Class 12 Maths notes |
Working of Fuel Cells
A fuel cell is a type of cell that converts chemical energy into electrical energy. It works on the principle of redox reactions. To continue reaction and generate electricity, a fuel cell requires a continuous supply of fuel (hydrogen) and oxidant (oxygen). The hydrogen is oxidized at the anode, while oxygen is reduced at the cathode. The reactions are:
- Anode: (oxidation).
- Cathode: (reduction).
- Overall Reaction: .
Electrons flow from the anode to the cathode through the external circuit, generating current. The electrolyte (e.g., NaOH solution) conducts ions.
Related Topics:
| NCERT Class 11 Notes | |
| Class 11 Chemistry NCERT notes |
Role of Porous Electrodes
A porous electrode is used to enhance the performance and efficiency of an electrochemical cell. It provides a large surface area for more electrochemical reactions to occur at the same time, which will increase the efficiency of the cell.
A porous electrode allows the flow of gases easily and reaches the active site. Moreover, better contact is provided between electrolyte, reactants and electrode material for better and efficient chemical reactions.
Types of Fuel Cells
Fuel cells are classified based on electrolyte and operating conditions. Below are the types of fuel cells.
- Proton Exchange Membrane Fuel Cell (PEMFC)
-
- Known as proton exchange membrane (polymer electrolyte)
- Can operate between 60-100°C.
- It is highly efficient and compact.
- Used in vehicles.
- Alkaline Fuel
- Uses aqueous potassium hydroxide (KOH) as electrolyte.
- Can operate between 50-85°C.
- Uses hydrogen and oxygen
- Alkaline is sensitive to CO₂ contamination.
- Used on spacecraft.
- Phosphoric Acid Fuel Cell
- Liquid Phosphoric acid is used as an electrolyte
- The operating temperature is 150-200°C.
- Fuel Hydrogen-rich gas
- Phosphoric Acid fuel cells are reliable and tolerant to impurities.
- Applications are hospitals, stationary power generation, and offices.
- Molten Carbonate Fuel Cell (MCFC)
- The electrolyte used in MCFC is Molten carbonate salt.
- Can operate at a temperature of 600-700°C
- The fuel used is carbon monoxide, natural gas, and hydrogen.
- Molten carbonate fuel cell is used in industrial energy systems and power plants
- Solid Oxide Fuel Cell (SOFC)
- The electrolyte used is a solid ceramic material (zirconium oxide)
- The operating temperature of a solid oxide fuel cell is 650-999°C.
- Biofuel, hydrogen and natural gas are used as fuel
- Used in power (CHP) systems and large-scale power generation.
Thermodynamic Efficiency
Fuel cells are highly efficient, converting chemical energy directly to electrical energy without combustion. The efficiency ( ) is:
where is the Gibbs free energy change, and is the enthalpy change. For the reaction, can reach 60-80.
Advantage of Fuel Cells
The advantage of fuel cells is mentioned below.
- Eco-Friendly
- Modular and Scalable
- Continuous power supply
- High efficiency
- Versatile application
- Reduced Dependence on Fossil Fuels
- Quiet operation
- Renewable
- Zero emission
- Fast charging
Cell Potential and Nernst Equation
Applications of Fuel Cells
Illustration for JEE Main
Chemistry Electrochemistry Exam