A galvanic cell is like a battery that takes a natural chemical reaction without needing any extra energy input to generate electricity. When learning about electrochemical devices in Class 12, we need to know the importance of this energy conversion process. Today, we discuss and study the principle behind how a galvanic cell can convert chemical energy from spontaneous redox reactions into electrical energy.
Also Read:
| NCERT Class 12 notes | |
| NCERT Class 12 Maths notes |
- What is Galvanic Cell?
- Components of Galvanic Cells
- Diagram of Galvanic Cell
- Galvanic Cell Construction: The Components
- Working Principle of Galvanic Cells
- Cell Notation
- Standard Cell Potential
- Spontaneity of Reactions
- Nernst Equation
- Concentration Cells
- Applications of Galvanic Cells
- Difference between Galvanic Cell and Electrolytic Cell
- Illustration for JEE Main
What is Galvanic Cell?
A galvanic cell generates electricity through spontaneous oxidation-reduction reactions that release energy. This electrochemical cell is also known as a voltaic cell.
Unit 2 of Class 12 of NCERT Chemistry textbook defines it as,
“a galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical energy. In this device the Gibbs energy of the spontaneous redox reaction is converted into electrical work which may be used for running a motor or other electrical gadgets like heater, fan, geyser, etc.”
Note that Gibbs energy (G) is a thermodynamic potential that can tell us if the cell reaction is spontaneous or not. It helps us calculate the voltage that the Galvanic cell generates.
The change ΔG has the basic calculation, ΔG =ΔH−TΔS
where,
- ΔH is the enthalpy change
- T is absolute temperature, and
- ΔS is entropy
So, when ΔG is less than 0, the cell operates in a spontaneous manner. In this case, a galvanic cell like a battery will work.
But if ΔG is more than 0, then it requires external input of electrical energy. And if ΔG = 0, then the cell like a battery is considered dead.
Components of Galvanic Cells
A galvanic cell comprises:
- Anode: The electrode (negative) where oxidation occurs, releasing electrons.
- Cathode: The electrode (positive) where reduction occurs, consuming electrons.
- Electrolyte: Ionic solutions (e.g., ) that conduct ions.
- Salt Bridge: A U-tube with an electrolyte (e.g., KCl) that maintains electrical neutrality.
- External Circuit: Wires allowing electron flow from anode to cathode.
Also Read:
| NCERT Class 11 notes | |
| Chemistry Class 11 NCERT Notes |
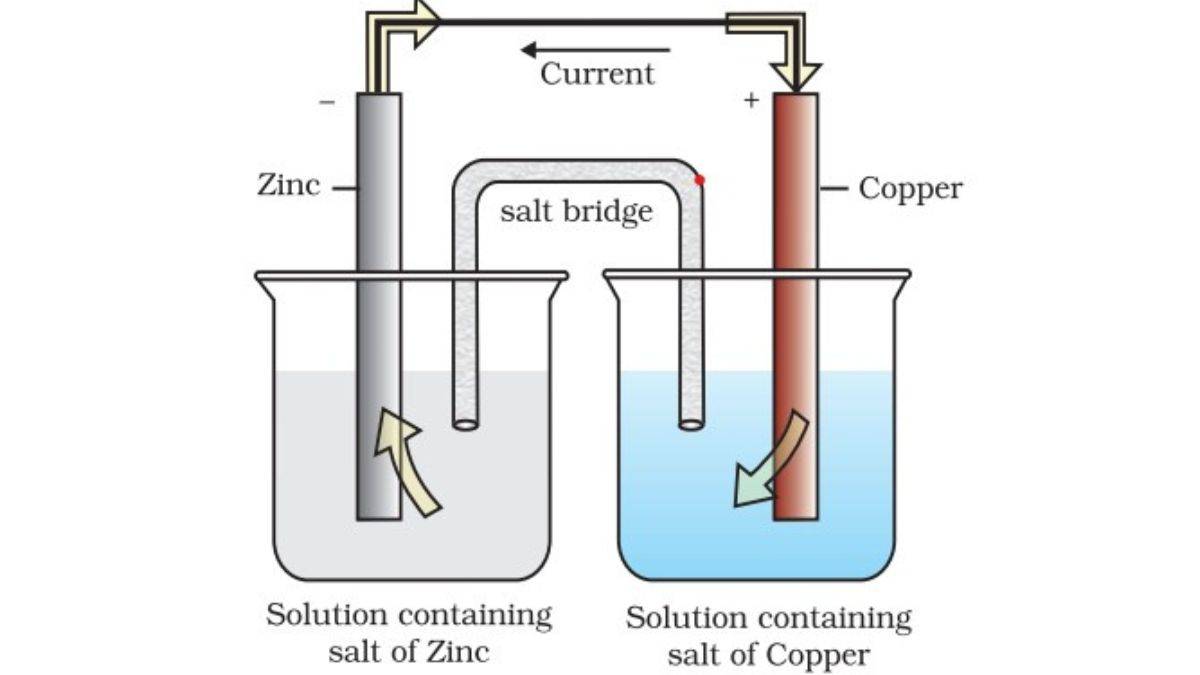
Diagram of Galvanic Cell
In a galvanic cell, there are two half-cells. Both of these have electrodes and electrolytes with internal and external connections. An external wire connects the two, while internally, there is a salt bridge that allows the flow of ions.
As we know, in electrochemical cells, electrons flow from the anode to the cathode, the same principle follows. In a galvanic cell, electrons from the place of oxidation (anode) to the place of reduction (cathode). This flow generates electricity. And that’s the primary application of a galvanic cell.
Let's go through the components of the Galvanic Cell diagram briefly, before understanding them in detail later in this article.
Also, it's important not to confuse the Galvanic Cell with Daniell Cell. In your textbook of Class 12 Chemistry, the diagram is of the Daniell Cell, which is a type of Galvanic Cell that uses a zinc electrode in a zinc sulphate solution and the copper electrode in a copper sulphate solution.
- There are two electrodes: Zinc and Copper. Zinc (Zn) is the anode that acts as the negative terminal. While Copper (Cu) is the cathode, working as the positive terminal here.
- The salt bridge is the one that is responsible for maintaining electrical neutrality. This makes the ion flow possible between the two terminals.
- As you see the external circuit above the two cells and salt bridge, it allows the flow of electrons from Zinc to Copper.
- We show the current direction of the circuit in the conventional sense, as it moves from positive charge to negative charge.
- There are two chemical reactions happening here. At the anode terminal, we have oxidation, which we represent as Zn -> Zn²⁺ + 2e⁻. At cathode, there is reduction, and we can show this as Cu²⁺ + 2e⁻ -> Cu .
Galvanic Cell Construction: The Components
After getting the basics of the diagram and its components, let's understand the setup. Below is the explanation of the galvanic cell construction.
-
Half Cells and Electrodes
A galvanic cell has two half-cells. These are redox couples, which we can define as oxidised and reduced forms of a pair of a substance. This pair is in the form of redox. One form is the oxidising agent and the other is the reduction agent.
Now, each of these half-cells has a metallic electrode. Both are separately dipped in an electrolyte solution that is of their individual metal ions.
On page 33, NCERT explains, “at each electrode-electrolyte interface there is a tendency of metal ions from the solution to deposit on the metal electrode”. At the same time, the “metal atoms of the electrode have a tendency to go into the solution as ions.”
-
Electrolytes and Salt Bridge
Both the electrolytes of the Galvanic cell have a salt bridge that’s connected internally. This bridge is used to maintain electroneutrality. And it’s important to use this kind of bridge, as it allows ions to have movement between the solutions. Otherwise, there would be a charge buildup to make the chemical reaction to stop.
-
Anode and Cathode Identification
The galvanic cell construction is designed in such a way that oxidation releases electrons at the anode. That’s what makes it have negative potential. On the other hand, reduction takes or consumes electrons at the cathode to make it have a positive potential.
Working Principle of Galvanic Cells
The galvanic cell working principle is based on the redox reaction. When the reaction takes place, chemical energy is converted to electrical energy. The redox reaction occurs in two different portions of galvanic cells, which are also known as two half reactions. The oxidation reaction occurs at the zinc electrode, while reduction occurs at the copper electrode.
In a galvanic cell, a spontaneous redox reaction drives electron flow. For a Daniell cell ( ):
- Anode Reaction: (oxidation).
- Cathode Reaction: (reduction).
- Overall Reaction: .
Electrons flow from the anode to the cathode through the external circuit, generating current. The salt bridge allows ion migration (e.g., to the anode, to the cathode) to prevent charge buildup.
Cell Notation
Galvanic cells are represented using standard cell notation: anode|anode electrolyte | | cathode electrolyte | cathode. For the Daniell cell, indicates Zn is indicated as the anode, Cu as the cathode, and || as the salt bridge. Phase notations (s, aq) are included.
Standard Cell Potential
The cell potential ( ) measures the driving force of the redox reaction. Standard cell potential ( ) is calculated under standard conditions ( atm):
where is the standard reduction potential. For the Daniell cell, , so:
Spontaneity of Reactions
Nernst Equation
Concentration Cells
Applications of Galvanic Cells
Difference between Galvanic Cell and Electrolytic Cell
Illustration for JEE Main
Chemistry Electrochemistry Exam