- What Are The Different Types of Electrochemical Cells?
- What is the Importance of Salt Bridge in Galvanic Cell?
- What are The Applications of Electrochemical Cells?
- What is The Significance of Salt Bridge in Galvanic Cell?
What Are The Different Types of Electrochemical Cells?
Electrochemical cells are devices that either generate electricity from chemical reactions or use electricity to drive chemical reactions. Following are the main categories based on whether they produce or consume electrical energy. Entrance exams such as IISER and IIT JAM ask conceptual questions based on the different types of electrochemical cells.
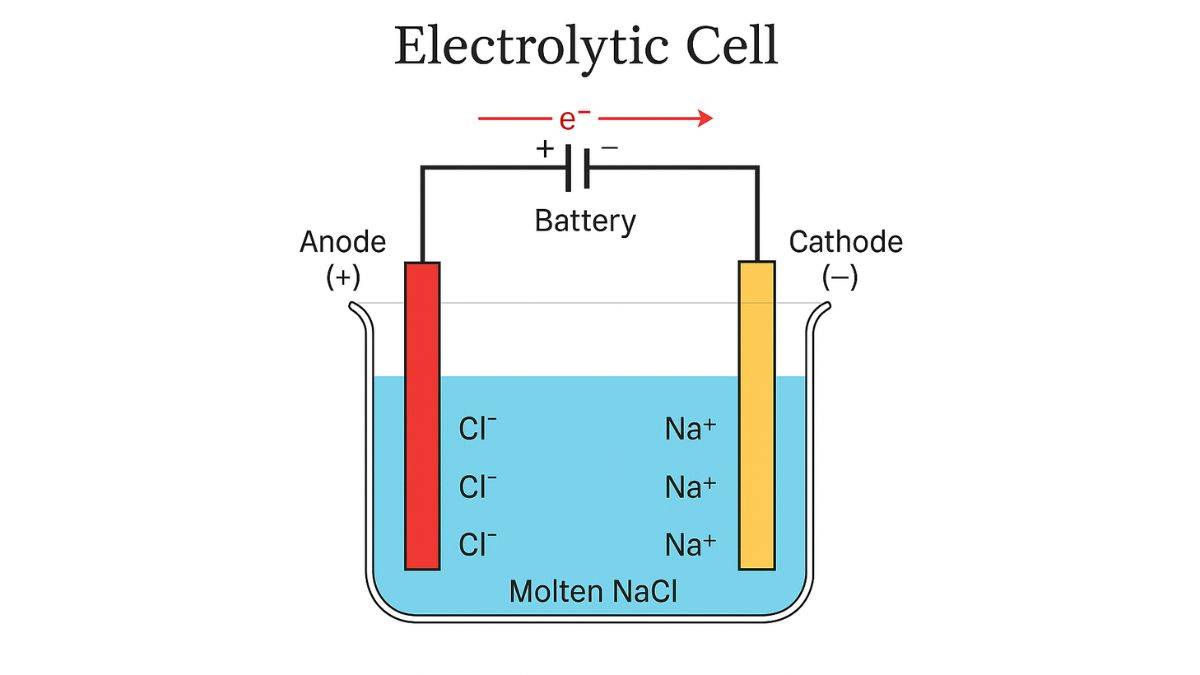
1. Electrolytic Cells
These electrochemical cells consume electrical energy to drive non-spontaneous chemical reactions. They are essentially the reverse of galvanic cells. These types of electrochemical cells operate on external energy. Here, external electrical energy forces electrons to flow in a direction opposite to what would occur naturally, driving otherwise unfavorable reactions.
2. Concentration Cells
These are galvanic cells where both electrodes are made of the same material, but they're in contact with solutions of different concentrations. The potential difference arises from the concentration gradient. For example, two copper electrodes in copper sulfate solutions of different concentrations will generate voltage. The electrode in the more dilute solution acts as the anode, while the one in concentrated solution acts as the cathode.
| Important Reads For Students |
|
|---|---|
| NCERT Class 11 Chemistry Notes | |
| NCERT Notes | NCERT Class 11 Notes |
What is the Importance of Salt Bridge in Galvanic Cell?
In case the electrodes are not internally connected, then cell will first produce electrical energy. However, in sometime the electrodes will become polarised. This will cause an accumulation of the ions that have opposing charges near electrode. The electrons flow. Or else sale will stop functioning. To ensure that electrical energy is being generated continuously, electrodes must be connected internally. This is done through salt Bridge.
Let us consider the example of potassium chloride (KCl) in the salt Bridge. Potassium ions (K+) will naturally move towards cathode, whereas chloride irons (Cl-) will hit towards anode. As they move, they will help in neutralizing some charged ions that are building up and causing issues. This will keep everything balanced and will allow the cell to generate electricity.
Without the presence of a salt bridge, you will have issues because the anode side will become positively charge, whereas cathode side will become negatively charge. This will lead to no flow of electron, which means there will be no more electricity.
Once a solid bridge is added, it will provide right counter – ions for maintaining electrical neutrality throughout the entire system. This will ensure that charges keep on flowing and the cell keeps on working.
What are The Applications of Electrochemical Cells?
The following points highlight the areas of applications:
1. Phone and Everyday Gadgets
Your phone is basically useless without its battery. Every time you open an app, take a photo, or text someone, there are chemical reactions happening in there, turning stored energy into the electricity that makes everything work. Through a charger, you are reversing those chemical reactions, forcing energy back into storage for later. It's like filling up a gas tank, except with electrochemical cells instead of liquid fuel.
Same thing happens with your laptop, tablet, AirPods, electric toothbrush - they are all running on battery. Even that tiny battery in your car remote that seems to last forever is doing the same thing, just on a much smaller scale. Questions based on the applications of electrochemical cells are covered in the NCERT excercises of the chapter.
2. Cars
If you drive, you're already using electrochemical cells even if your car runs on gas. While jumping someone's car, you are connecting two chemical power sources together. Electric cars like Tesla Model 3s you see driving around are powered by thousands of little battery cells working together. And hybrid cars like the Prius are constantly switching between gas and battery power, trying to figure out the most efficient way to move you down the road. Even those electric scooters scattered around downtown run on lightweight batteries.
3. Mining
It takes massive amounts of electricity to pull aluminum out of rocks. We're talking about so much power that aluminum plants are usually built right next to dams. Even for chrome-plated objects metal parts are put in chemical baths and run electricity through them to deposit that coating. Your kitchen faucet, car bumper, even some jewelry - it all goes through this process.
4. Electricity
6. Healthcare
What is The Significance of Salt Bridge in Galvanic Cell?
Chemistry Electrochemistry Exam