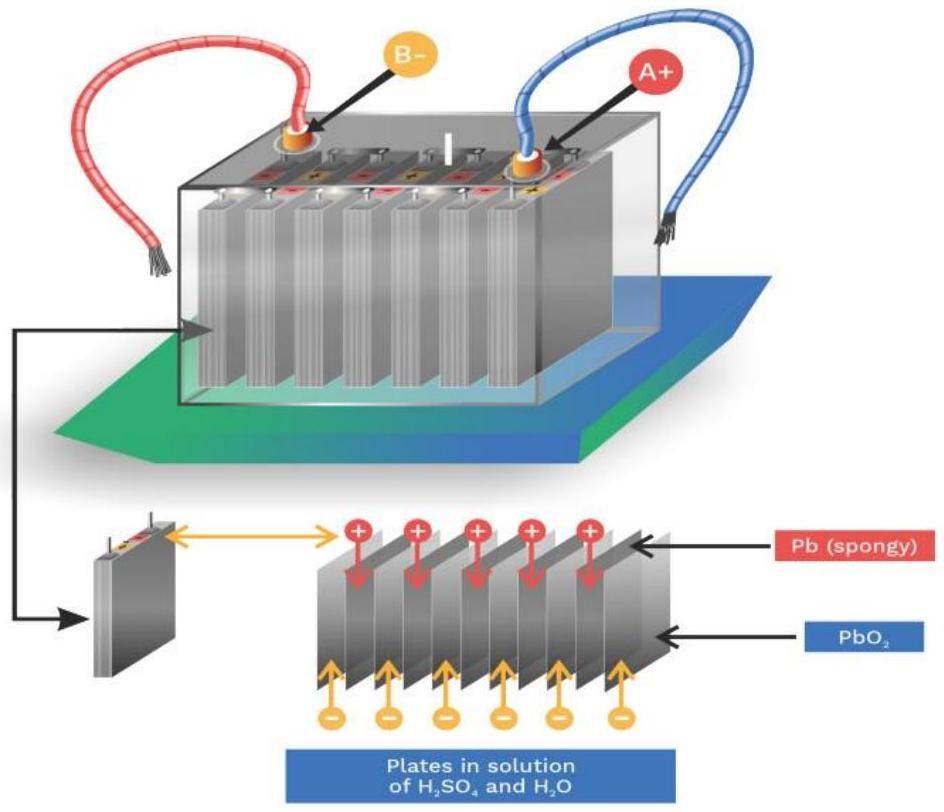
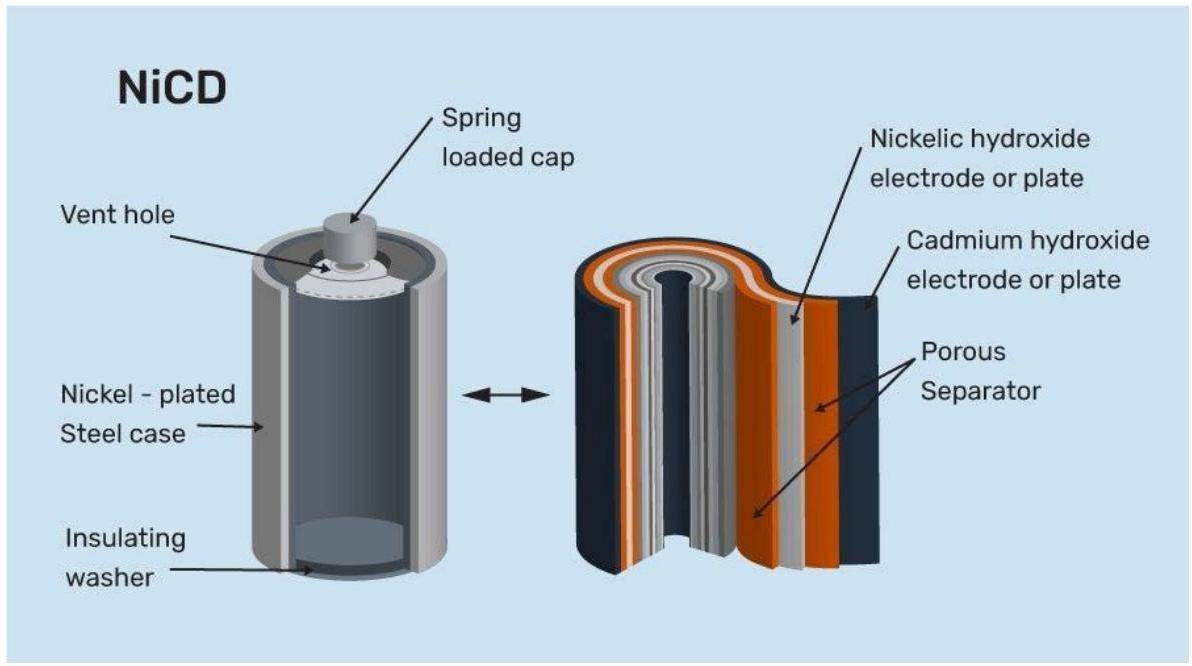
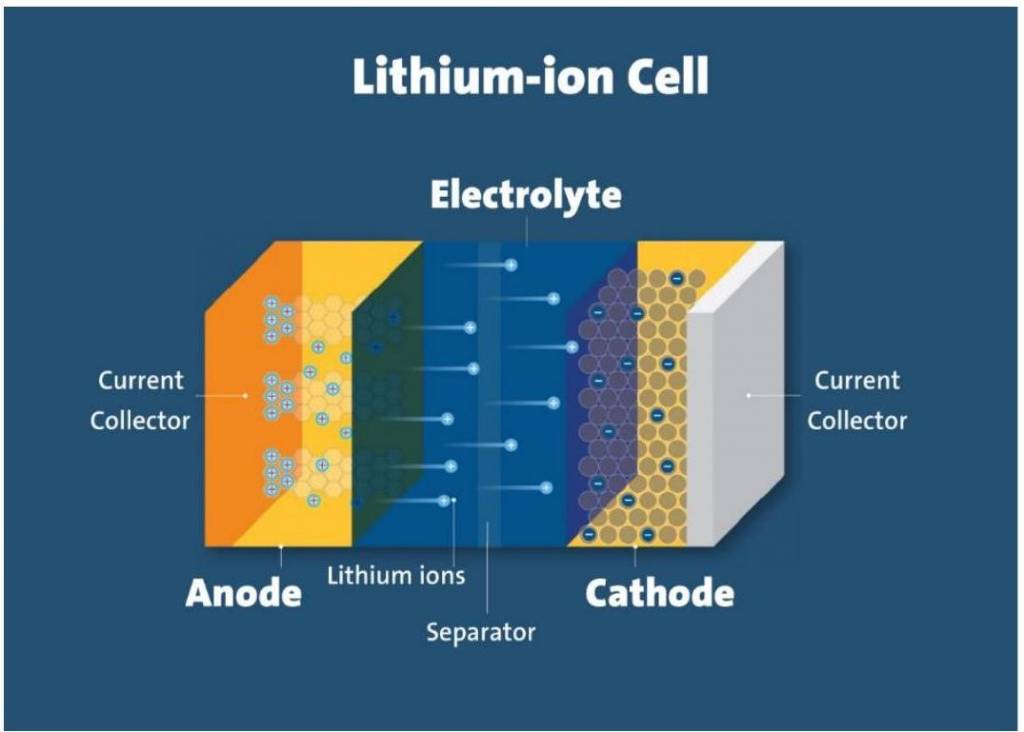
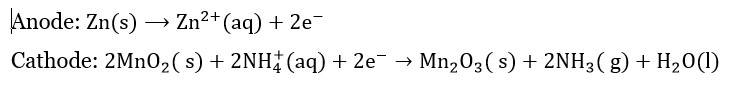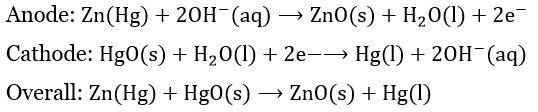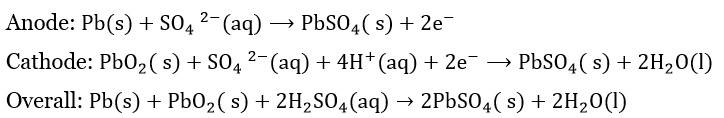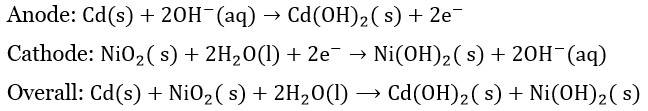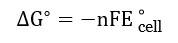Batteries are the most convenient source of electrical energy in today's world. Powering devices from a clock to the car, batteries have played a very crucial role. Batteries are electrochemical devices that work on the principle of redox reactions, where chemical energy is converted to electrical energy through the movement of electrons. Moreover, it is a steady source of electrical energy. Batteries is an important topics in Electrochemistry. In this article, we will focus on different types of batteries and their practical uses.
Importance of Batteries
Batteries are capable of providing electrical energy during a power outage. They are available in every size small to large, as per the usage. Batteries have a special place in our day-to-day activities. The essential characteristics of a battery are that it can be charged. Some of the practical applications of batteries are:
- Medical
- Health Instruments
- Constructions
- Military
- Vehicle
Students who are preparing for the CBSE exam can go through the article for better understanding of topics. The subject experts at Shiksha have prepared NCERT solutions for class 12 chapter 2 Electrochemistry focusing on the level of CBSE exam and entrace test like JEE Main, NEET, etc. Regulary practising NCERT solutions will improve the problem solving skills. Moreover, NCERT Class 12 Chemistry solutions for all chapter is available online.
- Batteries Definition
- Types of Batteries
- Battery Capacity
- Factors Affecting Battery Performance
- Properties of Batteries
- Uses of Batteries
- Examples of Batteries
Batteries Definition
As per the NCERT, the definition of batteries is " Any battery (actually it may have one or more than one cell connected in series) or cell that we use as a source of electrical energy is a galvanic cell where the chemical energy of the redox reaction is converted into electrical energy".
Explanation: A Battery is a source of energy where chemical energy is converted into electrical energy. An AA-type battery is an example of the galvanic cell.
Types of Batteries
Batteries are broadly classified into two categories:
- Primary Batteries: These are non-rechargeable batteries designed to be used until their chemical reactants are exhausted. After that, they are discarded.
- Secondary Batteries: These are rechargeable batteries that can be used multiple times. The chemical reactions is reversed by applying an external electrical source, regenerating the reactants.
Primary Batteries
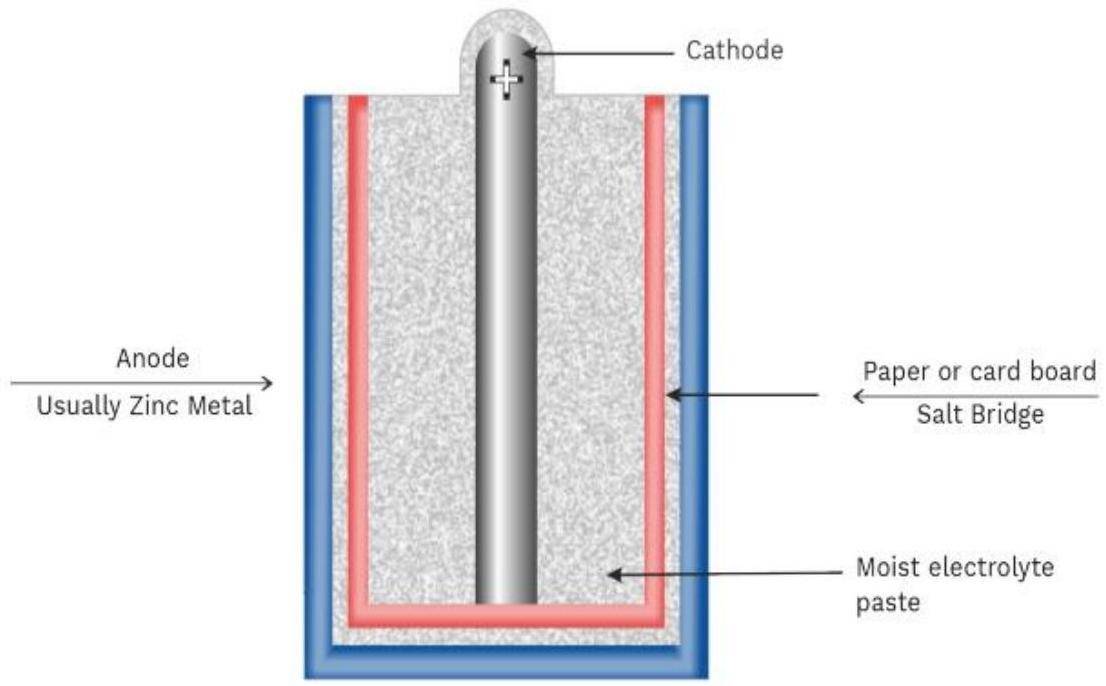
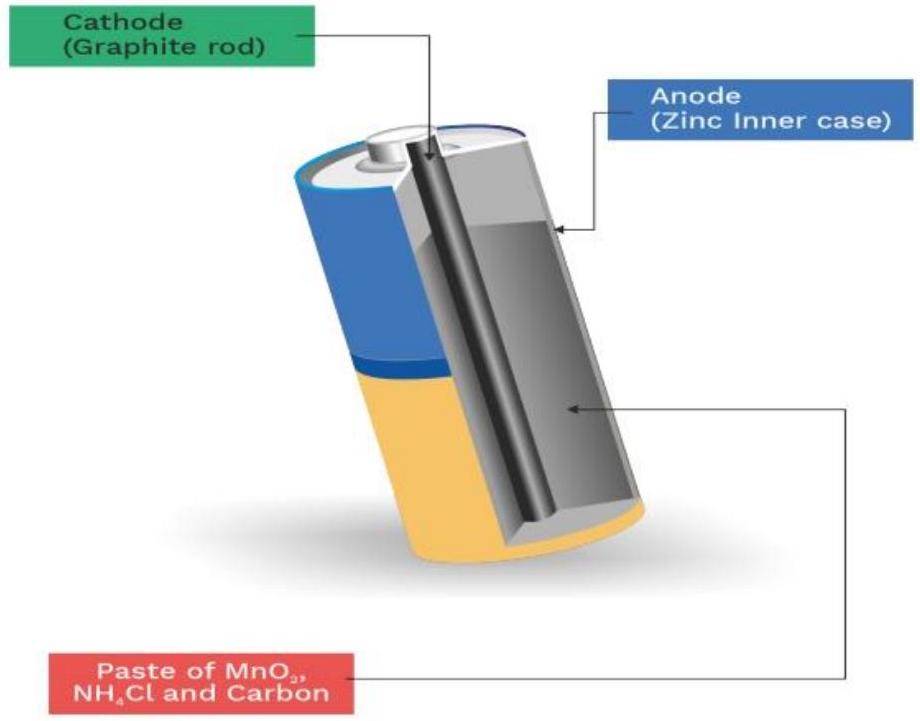
- Dry Cell (Leclanché Cell)
-
- The most common example of primary battery.
- Anode: Zinc container.
- Cathode: Carbon (graphite) rod surrounded by powdered manganese dioxide and carbon.
- Electrolyte: A moist paste of ammonium chloride and zinc chloride.
Cell Reaction:
- Voltage: Approximately 1.5 V.
- Uses: TV remotes, clocks, transistors, and toys.
Limitations:
- Voltage decreases with use.
- Cannot be recharged.
- The acidic ammonium chloride can corrode the zinc container over time, leading to leakage.
- A carbon graphite rod (cathode) is placed in the Zinc container, which acts as an anode
- The carbon graphite rod is surrounded by powered manganese dioxide and carbon.
- The space between electrodes is filled with a moist paste of ammonium chloride (NH4Cl) and Zinc Chloride (ZnCl2).
Mercury Cell
Battery Capacity
The capacity of a battery is the amount of electrical charge it can store. It is expressed in ampere-hour (Ah). For example, a battery with a capacity of 1 Ah, means it can provide a current of 1 ampere for 1 hour.
The knowledge of redox reactions, electrode potentials, and cell potentials (EMF) is important to understand batteries. The EMF of a battery is calculated using the standard electrode potentials of the half-cells involved.
Factors Affecting Battery Performance
- Temperature: Battery performance can be affected by the temperature. It may decrease at very high or very low temperatures.
- Discharge Rate: The higher discharge rates can reduce the battery's effective capacity.
- Age: The capacity of the battery gradually decreases with age.
- Charging and Discharging Cycles: A battery has a limited charging and discharging cycles.
- Batteries are used in various equipment based on their properties and performance.
Properties of Batteries
Batteries are used in various equipment based on their properties and performance. The properties of batteries are mentioned below.
- Power Capability
- Life cycle
- Capacity
- Electrode materials
- Voltage
- Energy density
- Shelf life
- Charging current
- Safety
Uses of Batteries
Battery Use in Vehicle
Now the world is moving to today's Electric Vehicles (EVs). These vehicles are powered by lithium-ion batteries. These batteries are rechargeable and have a good lifespan.
Uses of Batteries in Homes
Many devices in our home are equipped with disposable and reusable batteries. TV remotes, clocks, and torches use disposable batteries. While mobile phones, digital cameras, video games, etc. use reusable batteries. Moreover, inverters are used for power backup.
Uses of Batteries in Health Instruments
Engineers have developed various health instruments that are powered by batteries. Devices such as hearing aids, insulin pumps, pacemakers, artificial limbs and many other instruments use batteries for functions.
Examples of Batteries
Batteries are mainly of two types: primary and secondary. Below are examples of batteries and their usages.
| Types of Batteries |
Usage |
| Alkaline battery |
Flashlight, remote control, toys |
| Zin-carbon battery |
Clocks, transistors |
| Lithium battery |
Watches, calculator, cameras |
| Silve oxide battery |
Hearing aids, small electronics |
| Lead-acid battery |
Car batteries, inverters |
| Nickel-cadmium (NiCd) battery |
Medical equipment, power tools |
| Lithium-ion battery |
Mobile, laptops, EVs |
| Lithium-Polymer (Li-Po) |
Drones, RC vehicles |
Chemistry Electrochemistry Exam