Solubility refers to the ability of the substance (solute) to form a solution with another substance called the solvent. The solute may be solid, liquid or gas whereas solvent is mostly solid or liquid. Solubility is a fundamental concept in the study of solutions, homogeneous mixtures of two or more substances. Concept of solubility is not applicable whenever there are irreversible chemical reaction between two substances even if the two have been dissolved in one another. CBSE board class 12th science students must well-versed in the concept of solubility which is why it is recommended to practice the NCERT solutions of this chapter.
- Key Definitions
- Types of Solutions
- Factors Affecting Solubility
- Henry's Law
- Solubility Product
- Conclusion
Key Definitions
Solution
A solution is a homogeneous mixture where one substance (solute) is uniformly dispersed in another (solvent). A solution is a homogeneous mixture of two or more than two components. The component in the largest quantity is the solvent, determining the solution's physical state, and other components are solutes.
Solubility
Solubility is the maximum amount of solute that dissolves in a given amount of solvent at a specific temperature and pressure, often in grams per 100 grams of solvent. Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature, depending on the nature of solute and solvent, temperature, and pressure.
Saturated Solution
A saturated solution contains the maximum amount of solute that can dissolve at a given temperature and pressure, in dynamic equilibrium with undissolved solute. A saturated solution is one in which no more solute can be dissolved at the same temperature and pressure, in dynamic equilibrium with undissolved solute. The solute concentration in such a solution is its solubility.
JEE Main exam aspirants and NEET exam aspirants must prepare this topic thoroughly to perform better in the entrance examination.
Types of Solutions
Solutions are classified by the physical states of solute and solvent:
1. Gas in Liquid
A gas dissolves in a liquid until the rate of molecules entering the liquid equals the rate leaving it.
Examples: Oxygen (O₂) dissolves in water, supplying aquatic organisms with breathable oxygen; carbon dioxide (CO₂) dissolves in water under pressure to create carbonated beverages.
Governing Law: Henry’s law states that at constant temperature, the amount of gas dissolved is proportional to its partial pressure above the liquid:
C = kₕ × p
where C is solute concentration and kₕ the Henry’s constant.
Influencing Factors: Solubility decreases with rising temperature and increases with pressure. Molecular polarity and size also affect how readily gas molecules interact with solvent molecules.
2. Liquid in Liquid
When two liquids mix to form a single homogeneous phase, their miscibility reflects similar intermolecular forces.
Complete Miscibility: Ethanol and water mix in all proportions because both form strong hydrogen bonds, creating a uniform solution.
Partial or Immiscible: Gasoline (non-polar hydrocarbons) and water (polar) do not mix; however, gasoline and octane (both non-polar) form a single phase, illustrating the principle of “like dissolves like”.
Azeotropes: Some liquid–liquid pairs, such as ethanol–water, exhibit azeotropic behavior, where the mixture boils at a constant composition, complicating separation by distillation.
3. Solid in Liquid
A solid solute disperses at the molecular or ionic level within a liquid solvent, forming a homogeneous solution.
Examples: Glucose dissolves in water by forming hydrogen bonds with water molecules, yielding a clear sugar solution; sodium chloride (NaCl) dissociates into Na⁺ and Cl⁻ ions, which are stabilized by water’s polar dipole, creating brine.
Solvation Mechanism: Solvent molecules surround solute particles—ions or molecules—overcoming lattice or crystal energies (ΔH > 0) if the entropic gain (ΔS > 0) and enthalpic interactions (ΔH < 0) make ΔG negative.
Temperature Dependence: For most solids, solubility in water increases with temperature due to enhanced molecular motion and entropy changes.
Factors Affecting Solubility
Factors Affecting Solubility
Solubility is influenced by the following factors, as per NCERT.
1. Nature of Solute and Solvent
Solubility depends on chemical compatibility, with polar solutes dissolving in polar solvents due to intermolecular forces. Polar solutes dissolve in polar solvents (e.g., NaCl in water), while non-polar solutes dissolve in non-polar solvents (e.g., naphthalene in benzene), following the "like dissolves like" rule.
2. Temperature
For solids, solubility typically increases with temperature if dissolution is endothermic; for gases, it decreases with rising temperature. For solids in liquids, solubility increases with temperature if dissolution is endothermic (Δ_"sol " H>0) and decreases if exothermic ( Δ_"sol " H<0). For gases, solubility decreases with temperature due to exothermic dissolution, per Le Chatelier's principle.
3. Pressure
Pressure significantly impacts gas solubility in liquids, increasing with higher pressure. It has a negligible effect on solid solubility in liquids due to their incompressibility. For gases, solubility increases with pressure, as described by Henry's law.
Henry's Law
General Definition: Henry's law states that the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the liquid at constant temperature. NCERT Definition: At a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid or solution, expressed as: where is the partial pressure, is the mole fraction of the gas in solution, and is the Henry's law constant (NCERT, p. 41).
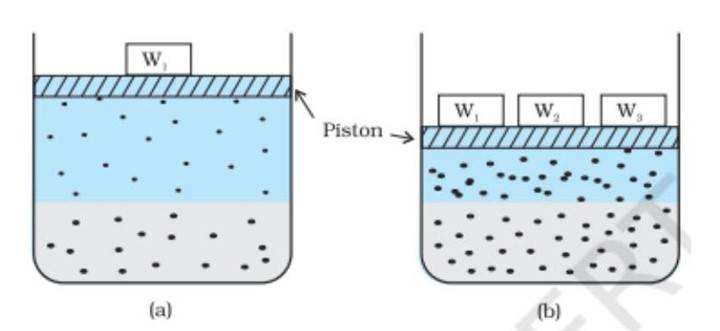
This figure illustrates the effect of pressure on gas solubility, showing (a) equilibrium at pressure , and (b) increased gas solubility at higher pressure.
Figure 1: Effect of pressure on gas solubility (NCERT, Fig. 2.1, p. 41).
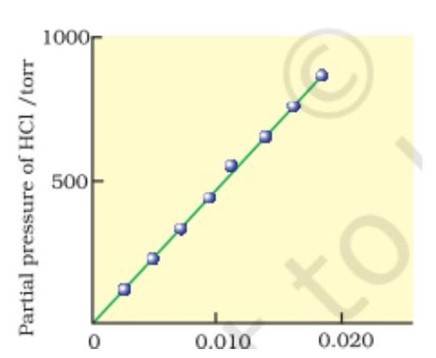
Graphical Representation: A plot of partial pressure versus mole fraction of a gas in solution yields a straight line, with the slope as (NCERT, p. 41).
This figure shows a linear plot of partial pressure versus mole fraction, illustrating Henry's law.
Figure 2: Plot of partial pressure versus mole fraction of a gas in solution (NCERT, Fig. 2.2, p. 41).
Example (NCERT, p. 42): Calculate millimoles of N2 in 1 L of water at 293 K with a partial pressure of 0.987 bar , given .
Moles of water in . Thus:
Applications (NCERT, p. 42): - High-pressure CO2 in soft drinks. - Helium-diluted air for scuba divers to prevent bends. - Low oxygen solubility at high altitudes causing anoxia.
Solubility Product
General Definition: The solubility product ( ) is the equilibrium constant for a sparingly soluble salt's dissolution, representing the product of ion concentrations. NCERT Context: For AgCl :
If solubility is (implied in NCERT for qualitative analysis).
Example (NCERT Exercise 2.27, p. 62): For CuS, . Calculate maximum molarity.
Intext Questions (NCERT, p. 43)
1. If H2S solubility in water at STP is , calculate Henry's law constant.
2. For CO2 KH = 1.67 × 108 at 298 K , calculate CO2 quantity in 500 mL of soda water at 2.5 .
Conclusion
Solubility, as defined by NCERT, is the maximum solute dissolvable in a solvent at specific conditions, influenced by nature, temperature, and pressure. Henry's law and solubility product calculations, supported by NCERT figures (Figs. 2.1, 2.2, p. 41), provide quantitative insights. These concepts are vital for Class 12 students to master solution chemistry.
Chemistry Solutions Exam