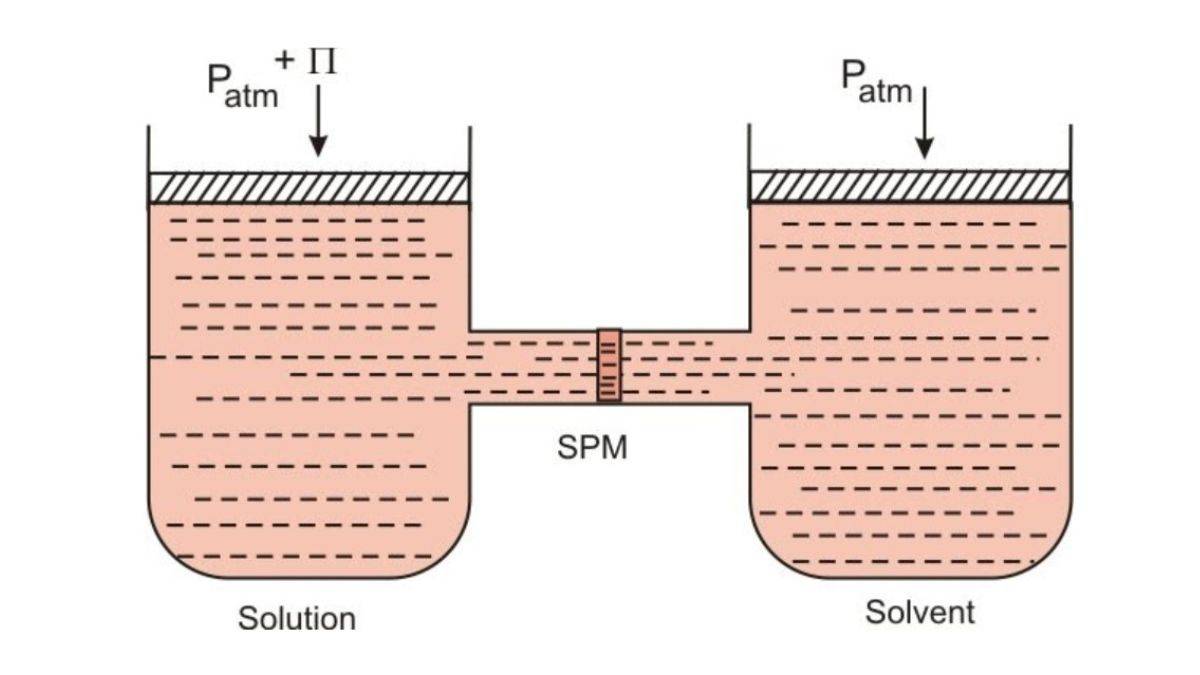
Osmotic Pressure is a vital concept in chemistry. This concept is defined as an external pressure applied to the solution to prevent the flow of pure solvent to the solution side across the semipermeable membrane. Osmotic Pressure is used to understand cellular functions, solution behaviour and biological membranes.
The osmotic pressure is dependent on the concentration of solutions. The interesting factor is that the solvent molecules always move from a lower concentration to a higher concentration of solutions.
Osmosis and Osmotic Pressure are important topics in Class 12 Chemistry Chapter 1 Solutions. In this chapter, students will learn the definition of Osmotic pressure, Van't Hoff's equation, and key points.
Also, students can find the NCERT Solution Class 12 Chemistry Chapter 1 Solution to practice questions on Osmosis and osmotic pressure. Solving the NCERT Solutions will boost confidence and help to understand the concept.
- What is Osmosis and Osmotic Pressure?
- Define Osmotic Pressure
- Osmotic Pressure: Van't Hoff equation
- Osmotic Pressure: Key points
- Applications of Osmotic Pressure
- What is Osmosis Pressure Examples?
- FAQs on Osmotic Pressure
What is Osmosis and Osmotic Pressure?
Osmosis is defined as the flow of solvent molecules from a pure solvent to a solution across the membrane. The external pressure applied to a solution to prevent the flow of pure solvent across a semipermeable membrane to the solution is osmotic pressure.
Define Osmotic Pressure
Osmotic pressure is defined as the minimum pressure needed to prevent the inward flow of a solution’s pure solvent through a semipermeable membrane. It is a colligative property of solutions which measures the pressure required to prevent a solvent's flow into a solution through a semipermeable membrane. It arises from the tendency of solvent molecules to move from an area of lower solute concentration to an area of higher solute concentration through a semipermeable membrane to equalise the concentration on both sides of the membrane.
Important Topics: NCERT Class 12 Chemistry Solution | NCERT Class 12 Physics Solutions
Osmotic Pressure: Van't Hoff equation
Dutch chemist Jacobus Van’t Hoff proposed the relationship between the osmotic pressure of a solution and the molar concentration of its solute. The mathematical relationship between osmotic pressure (π), solute concentration (c), and the gas constant (R) is described by the Van't Hoff equation, also known as the osmotic pressure formula.
π = icRT
Where:
- π is the osmotic pressure
- c is the molar concentration of the solute in solution (in moles per litre)
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
- i is the Van't Hoff factor.
Note that Van't Hoff equation is applicable for the solutions which behave like ideal solutions.
Osmotic Pressure: Key points
Semipermeable Membrane: Osmosis occurs through a semipermeable membrane that allows the passage of solvent molecules but not solute molecules. Common examples of semipermeable membranes include cell membranes and certain synthetic membranes.
Concentration Gradient: Osmotic pressure is directly proportional to the difference in solute concentration between the two sides of the membrane. The greater the difference in solute concentration, the higher the osmotic pressure.
Units: Osmotic pressure is typically measured in units of pressure, such as pascals (Pa) or atmospheres (atm).
Importance: Osmotic pressure is a crucial concept in biology and chemistry. In biological systems, it plays a role in processes like the movement of water in and out of cells. In chemistry, it is used to determine the molar mass of unknown solute particles in a solution through experiments.
Colligative Property: Osmotic pressure is considered a colligative property because it depends on the number of solute particles rather than their chemical nature. For example, a solution of sugar (a non-ionic solute) and a solution of salt (an ionic solute) will exert the same osmotic pressure if they have the same concentration of solute particles.
Applications of Osmotic Pressure
- Osmotic pressure is used in water purification. This process is applicable in wastewater remediation and extracting salt from seawater.
- Osmometry is used to determine the molecular mass of polymers.
- Osmotic pressure is commonly seen in plants. The leaves and stems of plants become dry and sag soon if they run out of water. In such cases, if we provide sufficient water, plants quickly absorb it and bulge. The process that causes this is osmosis, making water flow into the salt in plant cells. The cells, in turn, inflate and grow healthy. This is a continuous process observed during plant growth. As the cells absorb more water, they ensure healthy plant growth. Thus, osmotic pressure plays a key role in plant growth.
- Osmotic pressure plays an important role in maintaining cell homeostasis.
- The measurement of osmotic pressure is used to determine molecular weights of compounds.
What is Osmosis Pressure Examples?
Osmotic pressure occurs becase of osmosis. The real world example of osmotic pressure is mentioned below.
- Palnt Turgor (Maintaining Plant Structure)
- Food preservation
- Kidney Dialysis
- Reverse Osmosis (Water Purification)
- Dehydration and Rehydration in Humans
- Red Blood Cells in different solutions
FAQs on Osmotic Pressure
Q: At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars at the same temperature, what would be its concentration?
A: Here,
T = 300 K
π = 1.52 bar
R = 0.083 bar L
Applying the relation, π = CRT
where
π = osmotic pressure of solution
C = concentration of solution
R = universal gas constant
T = temperature
⇒C = π / RT = 1.52 / 0.083 X 300
⇒ C = 0.061mol/L
Concentration of the solution is 0.061mol/L
Q: Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27° C.
A:
Given-
Vant Hoff’s factor, i = 2.47
osmotic pressure, π = 0.75 atm
Volume of solution = 2.5L.
To determine the amount of CaCl2, we use vant Hoff’s equation for dilute solutions, given as,
πV = inRT
where, n is the number of moles of solute, R is solution constant which is equal to the gas constant and T is the absolute temperature.

Hence, the amount of CaCl2 dissolved is 3.425g
Q: Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25° C, assuming that it is completely dissociated.
A: Given-
Mass of K2SO4, w = 25 mg = 25 X 10-3 g,
Molar mass of K2SO4 = (39×2) + (32×1) + (16×4) = 174 g mol-1
Volume V = 2 liter
T = 250C + 273 = 298 K (add 273 to convert in Kelvin)
The reaction of dissociation of K2SO4 is written as,
K2SO4 → 2K + + SO42-
Number if ions produced = 2 + 1 = 3, hence vant Hoff’s factor, i = 3
Here, we use vant Hoff’s equation for dilute solutions, given as,
πV = inRT
where, n is the number of moles of solute, R is solution constant which is equal to the gas constant(0.082) and T is the absolute temperature (298 K).


Hence, the osmotic pressure of a solution is 5.27x10-3atm
Chemistry Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test