After studying integrated rate equations, we come on to the concept of temperature dependence in chemical kinetics. It has been scientifically proven that an increase in the temperature leads to a higher speed of the reaction rate. For example; increasing the temperature to about 10°C will double or triple the rate of reaction. In order to understand this concept, you need to be familiar with some key terms like rate constant, kinetic energy, barrier of activation energy etc.
This theory can be further explained through techniques like Arrhenius equations and collision theory, which the candidates can refer in the article given below:
Relevant Suggestion: NCERT Solutions
- What is Temperature Dependence?
- Arrhenius Equation
- Logarithmic Form
- Maxwell-Boltzmann Distribution
- Collision Theory
- Real Life Applications
- Class 12 Chemistry Notes for Important Chapters
- Class 12 Chemistry NCERT Solutions
What is Temperature Dependence?
Temperature dependence is a crucial factor in determining the rate of a reaction and is a frequently asked concept in competitive exams like JEE MAINS. According to this theory, the rate of a chemical reaction is directly proportional to the temperature i.e. a high temperature can lead to a faster rate of the chemical reaction. This is because increasing the temperature will lead to comparatively more collisions between the particles, which are necessary for speeding up the rate of the reaction.
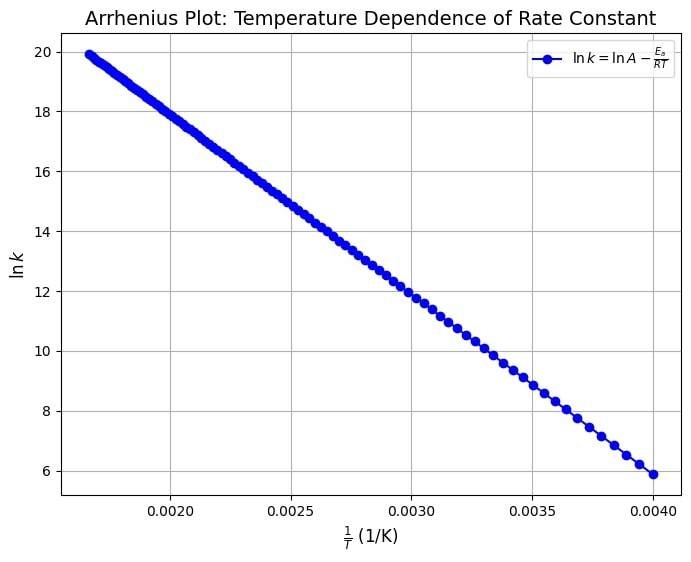
The arrhenius graph for temperature dependence can be depicted through the following image.
Arrhenius Equation
This is the formula which is used to depict how change in the temperature will affect the rate of a chemical reaction.
The temperature dependence of the rate constant ( ) can be given by the Arrhenius equation:
where:
: Rate constant.
: Pre-exponential factor (frequency factor), related to collision frequency and orientation.
: Activation energy ( ), the minimum energy required for a reaction.
: Gas constant ( ).
: Absolute temperature (K).
: Euler’s number
As the temperature T increases, the fraction will also grow, leading to a higher rate constant (k) and a faster speed of the reaction.
Logarithmic Form
Taking the natural logarithm of the Arrhenius equation:
This resembles a straight-line equation ( ), where plotting vs. yields:
Slope:
Intercept:
For two temperatures and with rate constants and :
Subtracting:
Converting to base-10 logarithm:
This equation is used to calculate or predict rate constant changes.
Maxwell-Boltzmann Distribution
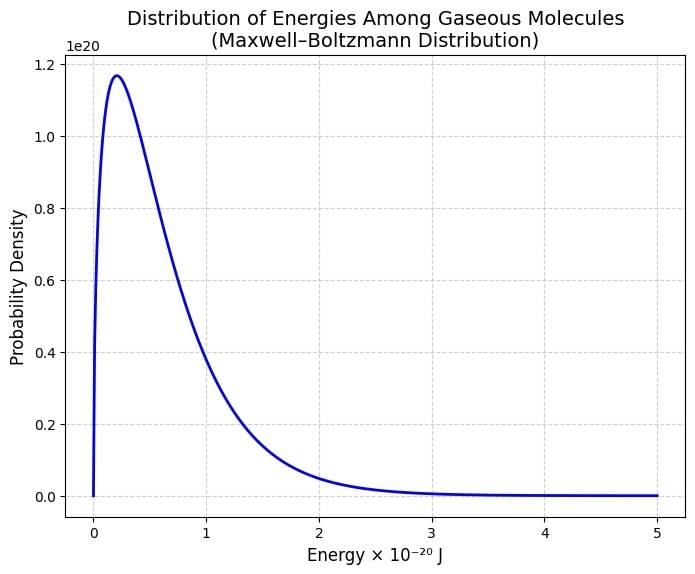
The Maxwell-Boltzmann distribution is a technique used to calculate the exact energies of different particles of a gas at a particular temperature. There are different molecules in a gas and each of them will have different speeds. Some will be fast and some will be slow.
Mathematical Representation:
There is a term called distribution function which is used to find particles with the desired speed range. The probability that a particular molecule has an energy E will be:
f(E)=π2((kT)3/21)E1/2e−kTE
Where:
f(E): Probability density function for kinetic energy
k: Boltzmann constant (1.38 × 10⁻²³ J/K)
T: Absolute temperature (K)
Collision Theory
The collision theory is a principle used in chemistry to understand the mechanism behind the occurrence of chemical reactions. For a particular reaction to take place, the particles need to collide with each other, and the higher energies these particles have while colliding with each other, higher will be the rate of the reaction.
Collision theory is mathematically represented by:
k=pZe−EaRTk = pZ e^{-\frac{E_a}{RT}}k=pZe−RTEa
Where,
p: steric factor (orientation probability, 0 < p ≤ 1)
Z: collision frequency
e−Ea/RTe^: fraction of collisions with sufficient energy
Some key factors necessary for a collision to occur are as follows:
1. Collision Frequency:
These are the number of particles which collide with each other in a particular time frame. This frequency depends on
- Temperature: High temperature will lead to higher kinetic energy of the particles, causing collisions with more frequency.
- Surface Area: More surface area will ensure that more number of particles will interact with each other to cause a collision.
- Concentration of Reactants: The volume of reactants also majorly determines the intensity of the collisions.
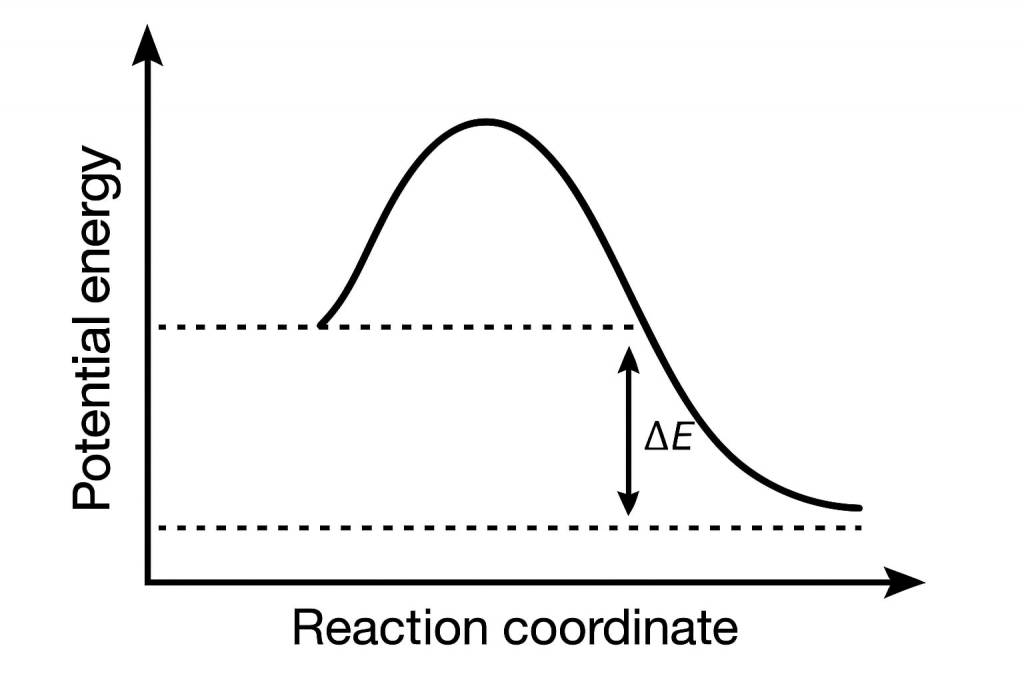
2. Activation Energy
This is referred to the minimum energy threshold required for a successful collision to take place. Particles will only collide with each other if their kinetic energy is more than the Ea (activation energy).
3. Orientation
According to this factor, the molecules must be in proper alignment with each other to produce a high frequency collision. This is determined by a sterlic factor p, which states that:
Rate∝p⋅Z⋅e−RTEa
Click to Read: NCERT Chapter 3 Chemistry: Chemical Kinetics
Real Life Applications
Here are some of the practical uses of this concept of temperature dependence in our daily lives:
- Heating and Cooking
- Preservation of Food Items
- Drug and Chemical Manufacturing
- Biomedical Sector
- Environmental Science
- Fuel Combustion
- Food Industry
- Pharmaceuticals
- Biological Enzymes
- Electrical Appliances (such as a thermometer)
Class 12 Chemistry Notes for Important Chapters
The notes given below are designed with the goal of helping candidates in their preparation for high level competitive exams:
| Chapter No. | Chapter Notes |
|---|---|
| 1 | The Solid State Notes* |
| 2 | Solutions Notes |
| 3 | Electrochemistry Notes |
| 4 | Chemical Kinetics Notes |
| 5 | Surface Chemistry Notes* |
| 6 | The p-Block Element Notes* |
| 7 | The d- and f-Block Elements Notes |
| 8 | Coordination Compounds Notes |
| 9 | Haloalkanes and Haloarenes Notes |
| 10 | Alcohols, Phenols and Ethers Notes |
| 11 | Aldehydes, Ketones and Carboxylic Acids Notes |
| 12 | Organic Compounds Containing Nitrogen Notes |
| 13 | Biomolecules Notes |
| 14 | Polymers Notes* |
| 15 | Chemistry in Everyday Life Notes |
Class 12 Chemistry NCERT Solutions
Commonly asked questions
Why can two different reactions have different rates at the same temperature?
This happens because although they have the same temeprature constant, some of their other properties can also vary such as activation energies, surface area of the reaction, concentration of the reactant, etc. As a result, the rate of a reaction comes out to be different for the reactions because of their dependence on these factors.
What is the key difference between endothermic and exothermic reaction?
Endothermic reaction is a reaction which absorbs heat energy from the surrounding, making the environment cooler. The products have higher enthalpy than the reactants in this reaction.
On the other hand, Exothermic reaction is a reaction which releases heat energy into the surrounding, making the environment warmer. The products will have lower enthalpy than the reactants in this reaction.
How is the concept of temperature dependence used to generate high production by the industries?
Industries manily select an optimal temperature which is high enough to increase the reaction rate (molecules colliding with each other at higher fequencies) and at the same time is balanced to avoid any side effect. This effectively yields high quality production without any wastage or leading to harmful results while ensuring safety of the consumers too.
Chemistry Chemical Kinetics Exam