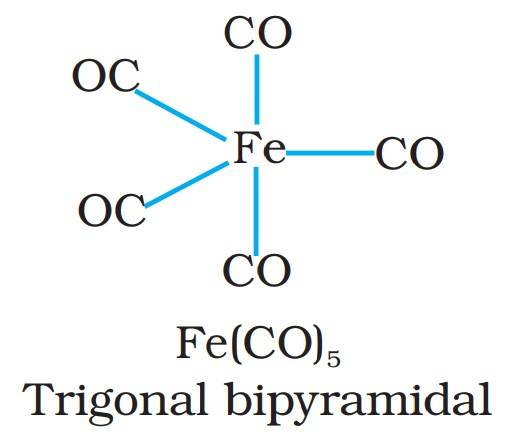
The metal carbonyls are a type of organometallic compounds that contain transition metal and carbonyl ligands. Most of the transition metals bind with carbon monoxide (carbonyl group) to form metal carbonyls. Metal carbonyls have a metal center and CO ligands are bound around it in specific angles. It gives metal carbonyls a specific geometrical shape, such as Tetracarbonyl-nickel is tetrahedral, and Pentacarbonyl-iron, which is trigonal bipyramidal.
In 1984, Ludwig Mond formed the first known metal carbonyl Ni(CO)₄ by heating the nickel powder in carbon monoxide steam (Mond refining process). Metal carbonyls are a special class of coordination compounds (containing metal-carbon bonds). The metal and carbon form a bond in metal carbonyls through the synergic bonding process.
The metal-carbon bond in metal carbonyls shows both σ and π characters based on the nature of the donation of a lone pair of electrons. Our NCERT Notes cover various concepts related to the metal carbonyls, including synergic bonding, formation, stability, structure, and examples of metal carbonyls are discussed below.
- What are Metal Carbonyls?
- Bonding of Metal Carbonyls
- What makes Carbonyls Ligands Different from Others?
- What is Synergic-Bonding?
- Structure of Important Metal Carbonyls
- Stability of Metal Carbonyls
- Applications of Metal Carbonyls
- Nature of Metal Carbonyls
- Complete Class 12 Study Material
- Frequently asked questions about Metal Carbonyl Bonding
What are Metal Carbonyls?
Metal carbonyls are a type of Organometallic compounds, where a transition metal, such as nickel, platinum, acts as the metal center and Carbon monoxide acts as a ligand group to form a metal-carbon bond.
These metal carbonyls are coordination compounds with a simple and well-defined structure. Moreover, metal carbonyl is also called homoleptic carbonyl since it uses only one type of ligand, which is CO. Homoleptic means it has same type of ligand attached to the central metal atom.
Let’s see a few metal carbonyl examples:
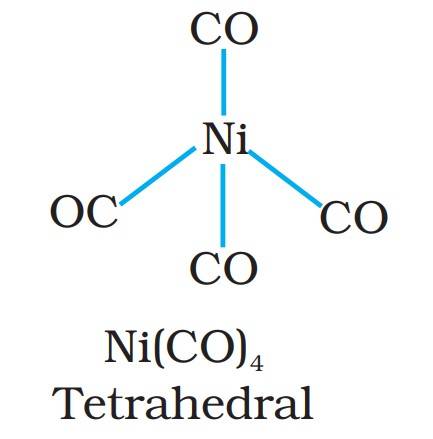
- Ni(CO)₄: This metal carbonyl has nickel as the metal center and 4 CO ligands around it, forming a tetrahedral structure.
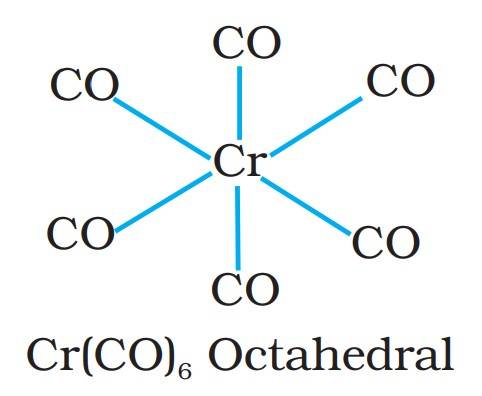
- Cr(CO)₆: This metal carbonyl has chromium as the metal center and 6 CO ligands around it, forming an octahedral structure.
Metal carbonyls form through the process of donation of a lone pair either from the metal to the CO atom or from the CO atom to the metal atom. These metal carbonyls need not always be mononuclear as in the above examples, they can also be polynuclear such as dimanganese decacarbonyl. The standard form to denote metal carbonyls is:
Do you know: What are organometallic compounds?
As explained above, metal carbonyls belong to organometallic compounds. Organometallic word itself gives meaning ot it. It is made by combining 'Organo', which refers to organic compounds and functional groups and 'Metallic' means including metal. So the complex compounds are made by combining a metal with an organic functional group. The main feature of organometallic compounds is the metal-carbon bond.
Bonding of Metal Carbonyls
The bonding in metal carbonyls is a result of synergic interaction between the CO ligand and the metal atom. Either CO donates a lone pair of electrons to empty metal orbitals known as σ-donation or the filled d-orbital of the metal atom donates a lone pair of electrons to the CO ligand known as π-back donation.
So, the metal-carbon bond in metal carbonyls can possess both σ and π character based on the electron lone pair donation process. A lone electron pair is a pair of electrons that does not take part in the regular bonding process.
There are 3 main aspects that need to be discussed related to metal-carbon bonding in metal carbonyls.
- How does the MC (Metal-carbon bond) form in metal carbonyls?
- Explanation of the metal-carbon bond: Valence bond theory
- Explanation of the metal-carbon bond: Molecular Bond Theory
How does the MC (Metal-carbon bond) form in metal carbonyls?
Most of the transition metals form a coordination compound with a carbonyl ligand by following the 18-electron rule. They do this to achieve a state of higher thermodynamic stability, and the electronic configuration becomes similar to noble gas.
What is the 18-electron rule?
The 18-electron rule states that the sum of electrons present in the d-orbital of the metal center and electrons donated by the surrounding ligand must be 18.
Metal carbonyls have both types of bonding, σ-bond and π-bond. What is the role of the carbonyl molecule and the transition metal in forming these bonds? Let’s discuss;
What does the CO ligand do?
The CO Ligand has the 5σ highest energy occupied molecular orbital, which acts similarly to a lone pair of electrons. The carbonyl molecule donates this lone pair of electrons to the vacant d-orbital of the metal. This process gives rise to the σ-bond.
However, in the case of back bonding, the CO atom receives the lone pair of electrons from already filled d-orbitals of the metal atom. This is also known as synergic bonding, which we will discuss below. This process helps in the formation of a π-bond.
What does the transition metal (Metal center) do?
The empty d orbital of the metal center overlaps with the highest energy orbital of the CO ligand through gaining the lone pair of electrons. Now, metal acts as a Lewis acid, gaining a lone pair of electrons, forming a σ-bond.
In the back bonding case, the metal center donates a lone pair of electrons from a filled d-orbital to the vacant 2π* orbital of CO ligands to form a π-bond.
In simple words;
- CO Ligands act as Lewis bases, donating electrons, and the metal acts as a Lewis acid, accepting electrons to form a σ-character metal-carbon bond.
- In back bonding, the roles are reversed CO ligand accepts electrons, and the metal center donates electrons to form a π-character metal-carbon bond.
Valence Bond Theory Explanation of the Metal Carbonyl Bonding
As per the valence bond theory, the CO ligand is in sp-hybridization and the electronic configuration of O and C in the hybridized state is below.
As shown in the electronic configuration, the sp_x-hybridised lone pairs on both atoms remain non-bonding. These lone pairs are the reason why the CO ligand behaves uniquely and can donate a lone pair to the metal.
The VBT considers bonding in CO as hybridization, which means the metal center generates empty hybrid orbitals of the same energy. These empty hybrid orbitals get fulfilled with donated electrons by CO (carbon monoxide) atoms.
This makes CO a σ-donor ligand in valence bond theory.
Molecular Orbital Theory Explanation of the Metal Carbonyl Bonding
The molecular orbital theory explains the phenomena in a better manner. As per MOT, there are 10 electrons needed to form a molecular orbital of a carbonyl ligand; they already have 6 electrons. The electronegativity difference makes BMO and ABMO get different numbers of electrons.
The Bonding MO will have more influence of oxygen, while the antibonding MO will have more influence of carbon. The electron sharing in CO molecular orbital makes its bond order three.
Molecular orbital filling of Carbonyl ligand:
This theory better explains that lower empty molecular orbitals are used for accepting electron density from filled d-orbitals of the metal center. It also explains the weakening of the C-O bond in backbonding due to reduced bond order.
What makes Carbonyls Ligands Different from Others?
Carbon monoxide, as a carbonyl ligand, is a very unique ligand. Here are a few important points that make it a special ligand.
- The CO is an unsaturated carbonic compound, which makes it difficult for CO to σ-donate (electron) and accept π* electrons.
- The above property makes the CO ligand π-acidic (σ-base), which is why it acts as a Lewis acid, donating a lone pair of electrons to form a metal-carbon bond.
- Its π-acidic nature allows the CO ligand to have a strong field and greater d-orbital splitting.
- The π-electron donation in the case of π-back bonding is almost equal to the σ-donation from the metal to the CO ligand to form a σ-bond.
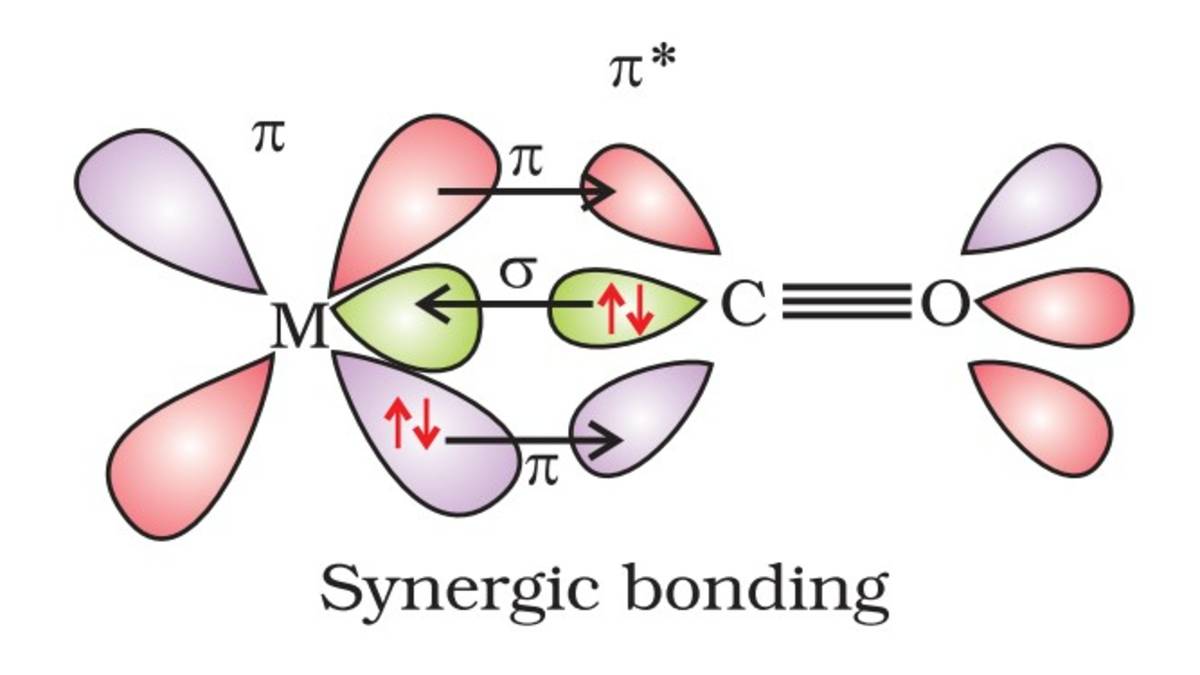
What is Synergic-Bonding?
Synergic bonding is the result of interaction between ligand and metal in a way where both can donate or accept a lone pair of electrons based on specific conditions.
In general, the ligands donate lone pairs of electrons to empty orbitals of transition metals to form organometallic compounds. However, when transition metals in metal carbonyls return, the process of donating lone pairs of electrons to form organometallic compounds (called back bonding) fulfills the conditions of synergic interaction.
In the metal carbonyls, this process of electron pair donation works two-way, meaning when the carbonyl ligand donates, it is called σ-donation, and when the metal donates to the ligand, it is called π-back donation.
σ Bonding in Metal Carbonyls
The Carbonyl ligand CO has a lone pair of electrons in the sp-hybrid orbital. This CO ligand donates its lone pair of electrons to the transition metal it forming a σ-bond. This σ-bonding is very similar to Lewis acid–base interaction; CO acts as a Lewis base, and the Metal center acts as a Lewis acid.
π Bonding in Metal Carbonyls
The Carbonyl ligand CO also participates in back bonding. The d-orbitals of transition metals get filled; these filled d-orbitals can donate electrons back into the empty π* (antibonding) orbital of CO. This is called π-back bonding.
This dual bonding interaction (σ-bonding and π-back bonding) is called synergic bonding.
Structure of Important Metal Carbonyls
The structure of metal carbonyls depends on multiple factors, including the number of ligand atoms and the transition metal center. We need to include the coordination number along with the geometry adaptation by the complex compound. You can check the structure of a few coordination compounds (We have given NCERT images for explanation) below;
MONONUCLEAR METAL CARBONYLS
Structure of Nickel tetracarbonyl
Structure of Chromium hexacarbonyl
Chromium Hexacarbonyl
Polynuclear Metal Carbonyls
These coordination compounds contain two or more metal atoms, with two metal-metal bonds and bridging CO ligands.
Examples:
- Dicobalt octacarbonyl (Co₂(CO)₈)
- Contains two CO atoms and 8 CO ligands
- Some COs are bridging between metals.
Types of CO Ligands in Structures
- The terminal CO ligand is bonded to a single metal center.
- The bridging CO ligand is shared between two or more metal atoms.
The coordination compound geometry is based on valence shell electron pair repulsion (VSEPR) and ligand electron count.
Stability of Metal Carbonyls
The Metal carbonyls are among the most stable coordination compounds. The reason for the stability of metal carbonyls is the strong metal-ligand bonding, due to synergic interaction between the transition metal (M) and the carbonyl ligand (CO). There are multiple factors that contribute to stability. Read below:
Factors Affecting the Stability of Metal Carbonyls
- Synergic Bonding: CO ligand denotes electron density to the metal via σ-donation, and the metal donates electron density back via π-back bonding. This metal-carbonyl interaction helps strengthen the metal-carbon bond.
- Low Oxidation State of Metal: The transition metals are generally available in low or even zero oxidation states. The CO ligand accepts electrons through π-back bonding to stabilize the low oxidation state. For example, Ni in Ni(CO)₄.
- 18-Electron Rule: The Majority of metal carbonyls follow the 18-electron rule, which gives a noble gas configuration, resembling the inert behaviour. This also leads to higher thermodynamic stability.
- Delocalization of Electrons in Polynuclear Carbonyls: In polynuclear complexes like Fe₂(CO)₉ or Co₂(CO)₈, delocalized bonding across metal-metal and metal-ligand bonds adds additional stability.
- Presence of Oxygen and Heat: The presence of heat of oxygen leads to weakening of the metal-carbon bond, which reduces the stability.
Example of Stable Metal Carbonyls:
- Cr(CO)₆ – Hexacarbonyl chromium(0)
- Ni(CO)₄ – Tetracarbonyl nickel(0)
- Fe(CO)₅ – Pentacarbonyl iron(0)
Applications of Metal Carbonyls
The key applications of metal carbonyls are mentioned below:
Catalysts in Industrial Reactions: Ni(CO)₄ and Fe(CO)₅ are widely used as catalysts in industrial processes.
Synthesis of Organometallic Compounds: The metal carbonyls are a kind of raw material to produce various organometallic compounds through synthesis.
Research in Bonding and Structure: These metal carbonyls are helpful to study metal-ligand bonding and coordination chemistry. Metal carbonyls are used in studying 18 elcetron rule and spectroscopic techniques.
Metal Purification: These complexes are very useful to purify the metal, such as Nickel carbonyl is used in the purification of nickel by the Mond process.
Material Science and Nanotechnology: These compounds are widly used in forming metal coatings through chemical vapor deposition and also to prepare nanoparticles of transition metals.
Nature of Metal Carbonyls
Complete Class 12 Study Material
Frequently asked questions about Metal Carbonyl Bonding
Commonly asked questions
How are metal-carbon bonds (M-C) formed in metal carbonyls?
The metal-carbon bonds (M-C) in metal carbonyls are due to the synergic interaction between the metal and carbonyl group. There are two types of bonding between metal and carbonyl group.
? -bond in Metal Carbonyls: The Carbonyl (CO) ligand donates a lone pair of electrons to the metal center to fill its empty d-filled orbital. This electron density donation is called? -donation.
? -back bond in Metal Carbonyls: This is the case of back bonding. The already filled d-orbitals return the electron density into the empty? * (antibonding) orbital of CO. This electron density donation by the metal is called? -back donation.
Both these combined? -bonding and? -back bonding are called synergic bonding.
What is the nature of bonding in NiCO4?
The nature of bonding in Ni (CO)? includes synergic bonding. The synergic bonding in metal carbonyls like nickel tetracarbonyl is due to two-way interaction of metal and carbonyl ligand electron density donation.
The synergic bonding is best explained thorugh Molecular Orbital Theory (MOT), which explains the donation of electron density from the filled d-orbitals of nickel (Ni) into the antibonding? * orbitals of the carbon monoxide (CO) ligand.
The synergic bonding can be described as a combination of? -bond formation due to donation of lone pair to metal center, and? back-bond formation due to donation of lone pair to CO ligand.
Why a carbonyl is a strong ligand?
There are multiple factors that make the carbonyl group a strong ligand. Check the list below for the reasons.
- Unlike other alkyl ligands, it is an unsaturated compound.
- Due to its unsaturated nature, it has difficulty donating? electron density.
- It has a tendency to accept? (Pie) antibonding electrons.
- CO ligand acts as Lewis acid and donates a lone pair of electrons to form a metal-carbon bond.
- The? -acidic nature of CO gives a strong field and greater d-orbital splitting.
Chemistry Coordination Compounds Exam