Many theories have been proposed to explain the chemical bonding in chemical compounds. The Chemical Bonding and Molecular Structure includes several theories, including VSEPR theory, Valence Bond Theory, and Molecular Orbital Theory. We have covered all these theories in our NCERT Notes with detailed conceptual explanations, exam-related facts, and more.
Molecular Orbital Theory (MOT) explains chemical bonding through the method of molecular orbital formation by combining atomic orbitals. This theory (MOT) explains major features of compounds such as magnetic properties, bond strength, delocalization of electrons (electron cloud), and others.
You can get complete information here on molecular orbital theory, including Linear Combination of Atomic Orbitals, Bonding and Antibonding, Bond strength, Bond order, Magnetic Properties, and other bond parameters. The short note PDF for this topic is also attached to this article.
- What is Molecular Orbital Theory?
- Postulates of Molecular Orbital Theory (NCERT Based)
- Sub-topics in Molecular Orbital Theory
- Formation of Molecular Orbitals
- Linear Combination of Atomic Orbitals (LCAO)
- Types of Molecular Orbitals
- Bond Order Calculation
- Magnetic Properties of Compounds
- Molecular Orbitals Diagrams of Important Compounds
- Applications in JEE Main
- CBSE Class 11 Study Material
- Class 11 Chemistry Chapter-wise Notes
- Frequently Asked Questions
What is Molecular Orbital Theory?
To understand MOT, we should dive into precursor theories a bit. The Valence Bond Theory (VBT) failed to explain a few key features, like paramagnetism in , Delocalized bonding in Benzene, non-existence of He₂, and others. F. Hund and R.H. Mulliken proposed Molecular Orbital Theory, focusing on addressing all these issues with VBT.
As per the Molecular Orbital Theory," Atomic orbitals consisting of delocalized electrons combine to form new molecular orbitals. An electron in a molecular orbital is influenced by two or more nuclei, depending upon the
number of atoms in the molecule (Polycentric). When two atomic orbitals combine, they form two molecular orbitals: Bonding and Antibonding molecular orbitals(MO). When the number of bonding MOs is higher than the number of antibonding MOs, a stable chemical bond forms."
Postulates of Molecular Orbital Theory (NCERT Based)
These key features(postulates) can help you get a complete picture of the MOT theory. Check the main features below.
Features of Molecular Orbital Theory
- The electrons are present in the various molecular orbitals in molecules, same as in atomic orbitals.
- Atoms with comparable atomic orbitals (energy and shape) combine to form molecular orbitals.
- A molecular orbital is polycentric, which means an electron in a molecular orbital is influenced by two or more nuclei.
- There are two types of molecular orbitals formed: Bonding and Antibonding molecular orbitals.
- The electrons in molecular orbitals are filled in the increasing order of orbital energy (from the lowest energy orbital to the highest energy orbital).
- The bonding molecular orbital has lower energy and higher stability in comparison to anti-bonding molecular orbitals.
- A molecular orbital explains the electron probability distribution around a group of nuclei in a molecule, similar to atomic orbitals.
- The molecular orbitals obey the Aufbau principle, Pauli’s exclusion principle, and Hund’s rule.
Sub-topics in Molecular Orbital Theory
To develop a good understanding, you need to learn a few major concepts of molecular orbital theory. We will discuss these topics in detail below. You can check the subtopics below.
- Formation of Molecular Orbitals
- Linear Combination of Atomic Orbitals (LCAO)
- Conditions for LCA
- Types of Molecular Orbitals
- Molecular Energy Diagrams
- Bond order calculation
- Prediction of Magnetic properties
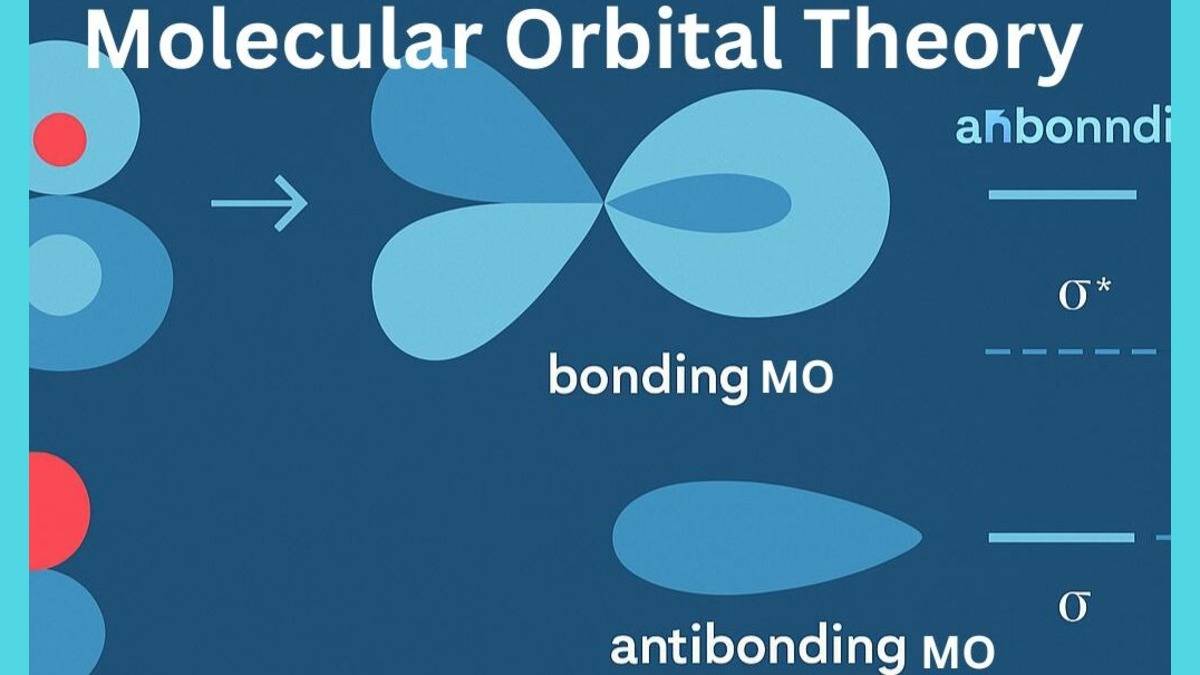
- Difference between Bonding and Anti-bonding Molecular Orbitals
Formation of Molecular Orbitals
The molecular orbital theory opened the path to understand the chemical bonding in a more detailed and precise way. This is important to understand how MOT theory explains the formation of molecular orbitals and subsequently the chemical bond due to these molecular orbitals.
As per the quantum mechanical model of atoms, all subatomic particles of the atom including electrons, behave in duality. All these electrons in atomic orbitals, apart from being sub-atomic particles, also behave like waves. When these electron waves of two or more atomic orbitals superpose, they interact with each other. These waves can either have constructive or destructive interference based on the phase difference.
When there is constructive interference of atomic orbitals, bonding molecular orbitals are formed. On the other hand, if the interference is destructive, then antibonding molecular orbitals are formed. However, there may be certain cases where nothing happens between two or more atomic orbitals a non-bonding molecular orbitals are formed. Check the summarized table below.
| Interference | Molecular Orbitals (MOs) |
|---|---|
| Constructive | Bonding MOs |
| Destructive | Antibonding MOs |
| No participation | Nonbonding MOs |
Linear Combination of Atomic Orbitals (LCAO) is the method used to explain the formation of molecular orbitals (MOs) from individual atoms (Atomic orbitals). It is very similar to the superposition of waves to form a new wave.
Linear Combination of Atomic Orbitals (LCAO)
The Linear Combination of Atomic Orbitals (LCAOs) explains the formation of the molecular orbitals, which later on become the foundation of the chemical bonding between the atoms. The formation of molecular orbitals can be understood in terms of the constructive or destructive interference of the electron waves of the combining atoms.
Conditions of LCAO
The LCAO, based on the conditions, is responsible for the formation of MOs. There are a few conditions of the LCAOs check below. These conditions are very useful for CBSE exams and other competitive exams.
- 1Atomic orbitals must have the same or at least comparable energy.
- 2 Atomic orbitals must have the same symmetry around the molecular axis.
- 3Atomic orbitals should overlap to the maximum extent.
Types of Molecular Orbitals
There are 4 major types of molecular orbitals classified based on their symmetry and overlapping extent. You can check the table below for detailed explanations and features of all these types of bonds.
| Bond | Specification |
|---|---|
| σ (Bonding) |
|
| σ* (Antibonding) |
|
| π (Bonding) |
|
| π* (Antibonding) |
|
Bond Order Calculation
Bond order measures bond strength and is calculated as:
Higher bond orders indicate stronger, shorter bonds.
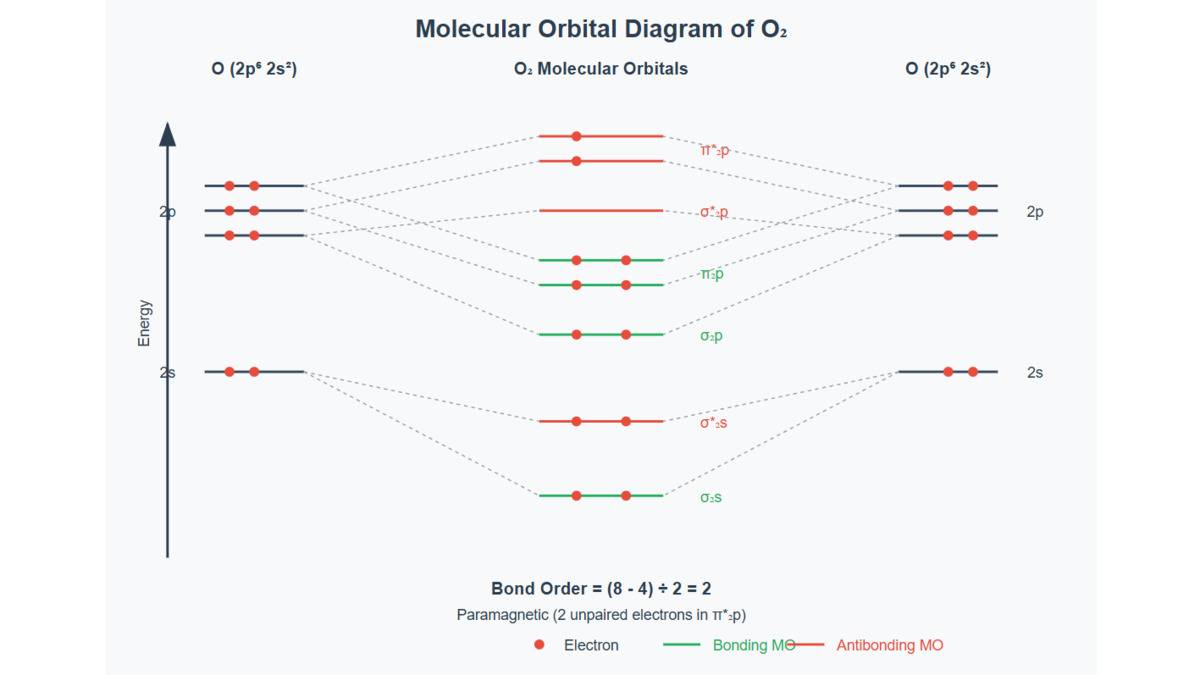
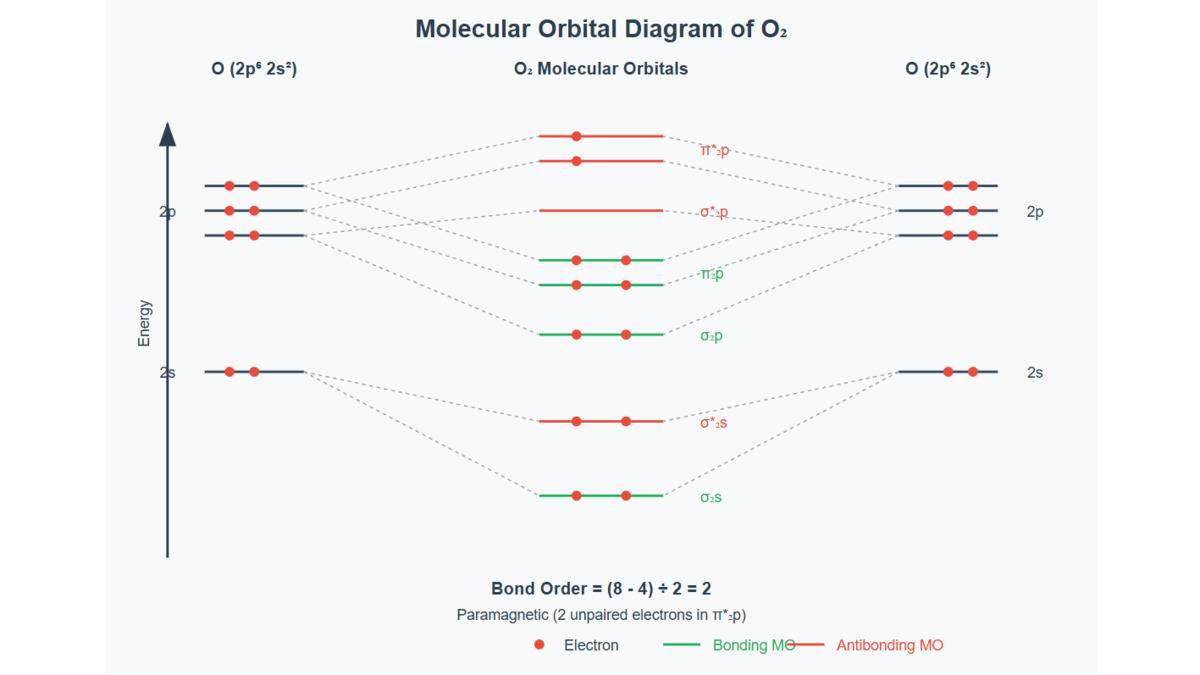
Example: For (14 electrons): - MO configuration:
- Bonding electrons: 10 ( 2 from from from from from )
Antibonding electrons: 4 (2 from from
Bond order: (triple bond)
This explains 's high stability (bond enthalpy ).
Magnetic Properties of Compounds
Molecular Orbitals Diagrams of Important Compounds
Applications in JEE Main
CBSE Class 11 Study Material
Class 11 Chemistry Chapter-wise Notes
Frequently Asked Questions
Commonly asked questions
What are the major differences between valence bond theory & Molecular Orbital Theory?
You can check the below given table for the comarative differences between valence bond theory and molecular orbital theory.
| Feature | Valence Bond Theory (VBT) | Molecular Orbital Theory (MOT) |
|---|---|---|
| Basic Concept | Overlap of atomic orbitals. | Atomic orbitals combine to form molecular orbitals |
| Bond Formation | Due to head-on (? ) or sideways (? ) overlap of atomic orbitals. | Linear combination of atomic orbitals (LCAO) to form bonding and antibonding MOs. |
| Explanation of Magnetic Behavior | Often fails to explain magnetism of molecules (e.g., O? is paramagnetic). | Accurately explains paramagnetism/diamagnetism (O? is paramagnetic due to unpaired electrons in antibonding orbitals). |
| Bond Order | Not clearly defined. | Bond order = ½ (No. of bonding electrons - No. of antibonding electrons) |
| Electron Delocalization | Electrons are localized between two atoms. | Electrons may be delocalized over multiple atoms. |
| Energy Consideration | Considers only overlapping orbitals and their energy. | Considers combination and energy differences of atomic orbitals. |
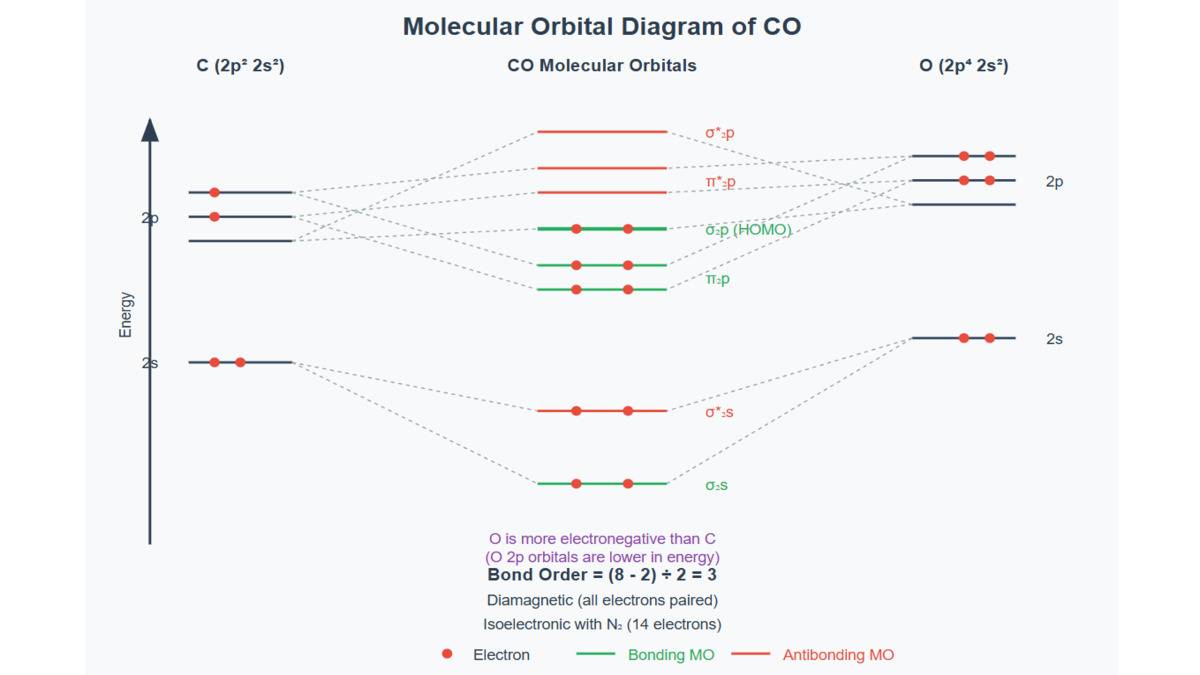
| Applicability | Works well for simple molecules like H? , HF, etc. | Better for explaining molecules like O? , N? , and ions like NO? , CN? |
Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics