Chemical bonding is a fundamental concept to understand the nature and formation of chemical compounds. Historically, many attempts were made to explain chemical bonding and molecular structure. Earlier theories, such as Kossel-Lewis approach and the VSEPR model, explain a few things, including molecular structure, dot structure. However, they could not provide a holistic theory for chemical bond formation.
Heitler and London initially gave the valence bond theory in 1927. Later, Pauling also added a few concepts and rectified it. VB Theory explains the formation of chemical bonds. The formation of atomic orbitals is explained using the electronic configurations of elements in this theory.
You will learn about atomic orbitals, hybridisation, types of hybridisation, resonance, and pairing of electrons between two atoms to form localized bonds.
Our NCERT Notes also cover other important topics of Class 11 Chemistry Chemical Bonding and Molecular Structure.
Candidates must have a clear understanding of various theories proposed to explain how the formation of coordination compounds takes place. By understanding VBT, you will move one step closer towards conceptual clarity. Read the complete article below:
- What is Valence Bond Theory?
- Postulates of Valence Bond Theory
- Formation of Covalent Bond: Orbital Overlapping
- Types of Orbital overlapping
- Factor Affecting Orbital Overlapping
- Types of Covalent Bonds
- Hybridisation in Valence Bond Theory
- VB Theory vs. VSEPR Theory
- Valence Bond Theory vs Molecular Orbital Theory
- Applications of Valence Bond Theory
- Limitations of Valance Bond Theory
- CBSE Class 11th Study Material
- Class 11 Chemistry Chapter-wise CBSE Notes
What is Valence Bond Theory?
The Valence bond theory (VBT) proposes to explain chemical bonds based on atomic orbitals and the electronic configuration of atoms. It primarily focuses on the formation of a covalent bond when half-filled atomic orbitals of two atoms overlap.
According to the Valance Bond Theory" Electrons in a molecule occupy atomic orbitals rather than molecular orbitals. When atomic orbitals of two atoms overlap, allowing their unpaired electrons to pair up, It results in a stable covalent bond."
The strength of the covalent bond formed depends on the extent of orbital overlap, which is maximized when orbitals are aligned correctly. This theory also explains the geometry of molecules.
Three important points, you must know about VBT are given below;
- The Covalant bond formation happens due to half-filled orbital overlap.
- Due to orbital overlapping having opposite signs, electron pairing takes place.
- The bond angles and molecular shape will be determined by the directional oriantation of the overlapping orbitals.
Read the article for a detailed view: Valence Bond Theory—Its Birth, Struggles with Molecular Orbital Theory, Its Present State and Future Prospects
Shaik, S., Danovich, D., & Hiberty, P. C. (2020). Prospects. Molecules, 26(6), 1624.
Postulates of Valence Bond Theory
The postulates of the Valence Bond Theory are essential to understanding the nature and formation of bonds. Belwo are the VBT postulates.
- Covalent bonds are formed due to the orbital-overlapping of two different atomic orbitals from two different atoms.
- Overlapping of orbitals increases electron density, hence increases the stability of the molecule.
- The presence of unpaired electrons in the valence shell of an atom determines the formation number of bonds with other atoms.
- The Pair of electrons in valence shells repel each other so that they minimise repulsion and maximise separation.
- The paired electrons do not participate in the formation of chemical bonds (valence bond theory).
- Covalent bonds are considered directional and are also parallel to the region corresponding to the overlapping atomic orbitals.
- Two types of covalent bond forms as per the valance bond theory: sigma (σ) and pi (π)
- Sigma bonds are formed from along the axis overlapping orbitals.
- Pi bonds are formed from sidewise overlapping orbitals.
Formation of Covalent Bond: Orbital Overlapping
What do we mean by overlapping? It means two entities, such as atom orbitals in the case of VB theory, occupy the same region of space. If we rephrase in technical terms, their wave functions overlap and share the electrons with both nuclei.
Why do Atomic Orbitals Overlap?
It is essential to understand the reason behind the atomic overlapping to completely understand VBT.
Atoms, like all isolated systems, tend to achieve the lowest possible energy state, which provides stability. Whenever any atom has half-filled orbitals, it tends to either share or transfer the unpaired electron to lower the total energy of the system.
How does Orbital-Overlapping Occur?
let's get into more details of the overlapping of half-filled orbitals;
- Electron Density Accumulation: When two atoms with half-filled orbitals come close, the unpaired electron accumulates between the two nuclie.
- Electrostatic Repulsion and Attraction:
- The attraction between accumulated electrons and nuclei increases gradually.
- The repulsion between nucleon-nucleon and electron-electron slowly reduces.
- The attraction outweighs the repulsion, creating a stable overlap.
- LCAO ( Linear Combination of Atomic Orbitals): This method provides a mathematical (quantum mechanical) explanation for the overlapping. It shows that constructive overlapping happens where the probability of increased electron density is higher.
- Condition for Overlapping - Pauli's Exclusion Principle:
- According to the pauli's exclusion principle, " overlapping obrbital must contain only one unpaired electron each with opposite spins. This allows two electron to occupy same spatial region of overlapped orbitals."
- The bond length must be such so that the attractive and repulsive forces are in balance,and offering maximum orbital-overlapping.
Formation of Covalent bond
In general there is two methods of overlapping which gives rise to covalent bonds. These overlapping forms two type of covalent bond: σ and π covalent bonds.
- Sigma (σ) bond: This bond is formed when two half-filled atomic orbitals overlap end-to-end, means head on on the same axis.
- Pi (π) bond: Unlike sigma bond, this is formed when two half-filled atomic orbitals overlap laterally or sideways.
Types of Orbital overlapping
There are majorly four type of orbitals overlapping discussed in NCERT textbooks. Understanding orbital overlapping type, their hybridization and impact on geometry is very important for JEE Mains and Advanced exam students. Here is a complete picture of all four type of overlapping.
-
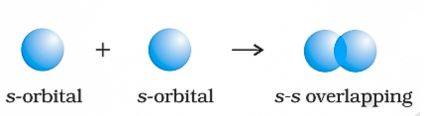
s - s Overlapping
s-orbitals are considered spherical. Two s orbitals overlap head-on along the internuclear axis as shown in below figure. This head on overlapping produces σ bond. The bond strength depends on the distance between nuclie and s-orbital size. A classic example of this is formation of hydrogen molecule. It forms single σ covalent bond between H - H atoms.
-
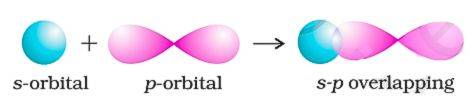
s - p Overlapping
One spherical s-orbital and one dumbbell-shaped p-orbital, of different atoms, overlap head-on along the internuclear axis in s-p overlapping (see the below figure). Hydrogen chloride (HCl) is a very good example to showcase the s-p overlap. In HCl, the H–Cl (σ) bond is formed by overlap of 1s orbital of H with 3p orbital of Cl.
-
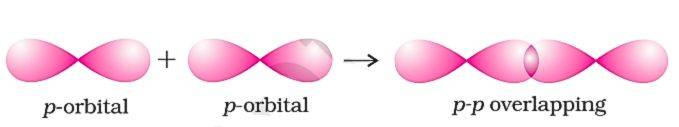
p - p Overlapping (Head on)
Two p-orbitals from different atoms overlap head-on along the internuclear axis, also called end-to-end overlapping. like other head on overlapping, It also a strong σ bond (covalent). In fluorine molecule, both Fluorine p-orbitals overlapp head on to form single F -- F bond.
-
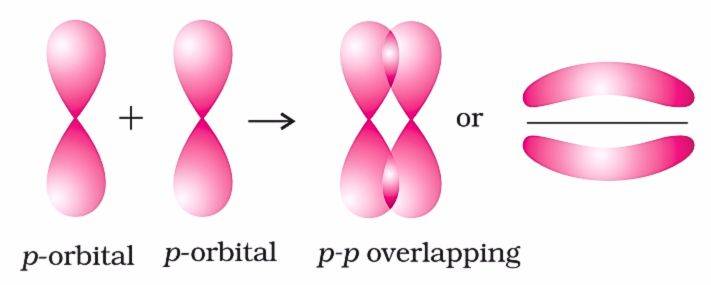
p - p Overlapping (Sideways)
In sideways or lateral overlapping, two p-orbitals overlap parallel to the internuclear axis (shown in the below figure). This type of overlapping creates π-bond. For example, the ethene (C=C)has two covalent bonds, one of them is σ bond, and another bond is formed due to the sideways overlapping of the unhybridized 2p orbitals.
Factor Affecting Orbital Overlapping
Here are several factors that affect the overlapping and bond strength. check below;
- Shape of Orbitals: The shape determines the extent of overlap, which results in stability and bond strength. Below is the order of orbital overlap based on shape: p-p (both dumbbell-shaped) > s-p (one sphere and other dumbbell-shaped) > s-s (Both spherical).
- Size of Orbitals: Smaller orbitals offer more compact and stable overlapping, forming stronger covalent bonds. You can phrase it as, "The larger the size of the orbital, the weaker the bond will be."
- Orientation of Orbitals: The orientation of orbitals is important to get maximum overlap. When two orbitals overlap along the internuclear axis (end-to-end), the overlapping region is maximum compared to other orientations. That is why axial overlaps form stronger σ bonds than π bonds formed by lateral overlaps.
- Directionality: The orbitals with directional character ( any shape other than a sphere which have a specific direction of internuclear axis), such as p, d, sp, sp² hybrids, give more effective overlap.
- Effective order of hybrid orbital overlap: sp > sp² > sp³ > sp³d
- Bond Length: The distance between atoms is important to know the extent of overlap. As we increase the distance between the two nuclei, the overlapping region decreases, and if we move closer, the repulsive force between the nuclei increases. Effective overlapping occurs at the equilibrium distance between atoms.
- Electronegativity: If atoms have the same or close electronegativity, they form a stronger covalet bond.
Types of Covalent Bonds
There are two types of covalent bonds: Sigma and Pi bonds. We classify the bond based on the overlap of the two orbitals. You can read about these two bonds in detail below;
Sigma ( ) Bonds:
When two atomic orbitals overlap head-to-head, they form a sigma bond. A sigma bond forms when head-to-head overlapping of atomic orbitals takes place. Due to head-on orbital overlap along the internuclear axis, there is increased electron density between the nuclei. This shared electron density gives rise to the strongest covalent bonds. The electrons participating in a σ bond are known as σ electrons.
Example: In , the 1s orbitals of two hydrogen atoms overlap along a straight line (head-on), so they form a sigma covalent bond.
Pi ( ) Bonds:
Unlike sigma bonds, Pi bonds form when p orbitals overlap sideways in the same phase. This overlap of atomic orbitals happens parallel to the internuclear axis. This parallel overlap of orbits creates electron density above and below the internuclear axis. this is how the π covalent bonds are formed. The π bonds weaker than the sigma bonds.
Example: In , each oxygen atom takes part in two overlaps. One sigma bond forms via p-orbital overlap, and one pi bond forms via sideways p-orbital overlap. So there is a double bond between oxygen atoms.
Hybridisation in Valence Bond Theory
As per the valence bond theory, the process of merging two atomic orbitals gives rise to a new hybridised orbital. This is called Hybridisation. There are several types of hybridisation. Check the table below:
| Hybridisation Type | Specification (No. of electron bond pairs & lone pairs) | Molecular Geometry |
|---|---|---|
| sp | 1 bond pair + 1 bond pair / lone pair | Linear ( apart). |
| sp² | 3 bond pairs / 2 bond + 1 lone pair | Trigonal Planar ( bond angles). |
| sp³ | 4 bond pairs/combinations with lone pairs | Trigonal Pyramidal ( bond angles). |
| sp³d | combinations of bond and lone pairs | Trigonal Bipyramidal (3D shape) |
| sp³d² | 6 bond pairs or with lone pairs | Octahedral (3D shape) |
| sp³d³ | 7 regions | Pentagonal Bipyramidal |
VB Theory vs. VSEPR Theory
Valence Bond Theory vs Molecular Orbital Theory
Applications of Valence Bond Theory
Limitations of Valance Bond Theory
CBSE Class 11th Study Material
Class 11 Chemistry Chapter-wise CBSE Notes
Commonly asked questions
Determine the hybridization and molecular geometry of Ammonia molecule.
The central atom of nitrogen has 5 valence electrons as per the electronic configuraion. During the formation of , 3 valence electrons forms three sigma bonds with hydrogen and one lone electron pair is left.
- The steric number of the ammonia molecule: SN=3 bonds +1 lone pair total electron domains.
- As per the steric number, there is hybridisation in ammonia.
- The lone pair causes repulsion, which leads to a trigonal pyramidal geometry with bond angles of .
Why can’t VBT explain delocalization or resonance?
The concept of delocalization or resonance can be explained for quantum mechanical atomic models in which electrons are considered to be spread over the entire molecular orbital.
The fundamental assumption of the valence bond theory contradicts the delocalization. The valence bond theory assumes that a covalent bond forms from the overlap of atomic orbitals on adjacent atoms, and electron density is localized between two specific nuclei.
That is why VBT cannot explain energy stabilization due to the resonance property.
How is orbital overlap maximum condition important for JEE exam calculations?
The valence bond theory explains the covalent bond formation for two half-filled orbitals. The bond strength depends on several factors, including the extent of overlap of the two atomic orbitals. The bond strength is directly proportional to the extent of overlap.
In simple words, the greater the amount of overlap between two orbitals, the stronger the covalent bond will be.
JEE asks many questions based on the comparison of bond strength for two different pairs of atomic orbitals forming a covalent bond. For example, why H–F bond stronger than the F–F bond?
Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics