- What is VSEPR Theory?
- Key Principles of VSEPR Theory
- Postulates of the VSEPR Theory
- Steric (VSEP) Number
- How to Determine Molecular Shape
- Molecular Geometries and Examples
- Factors Influencing Bond Angles
- Limitations of VSEPR Theory
- Applications of VSEPR Theory
- Study Materials for CBSE Class 11
- NCERT Class 11 Chemistry Chapter-wise Notes
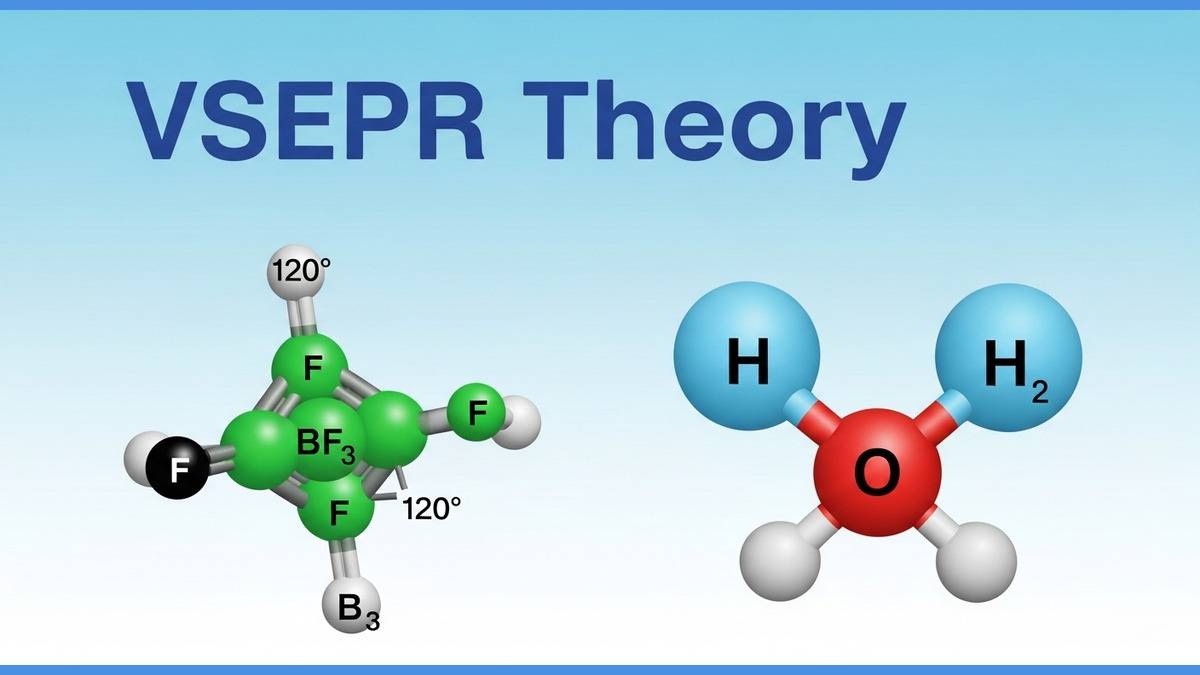
What is VSEPR Theory?
The Valence Shell Electron Pair Repulsion (VSEPR) theory is used to understand the molecular structure of chemical compounds. It takes the help of electron pair repulsion to predict the molecular structure. It is more useful in chemical compounds with covalent bonds.
The Valence Shell Electron Pair Repulsion (VSEPR) theory states that the molecular structure of a compound is determined based on the number of electron pairs around the central atom and the arrangement of electron pairs due to electrostatic repulsion between each other. These electron pairs tend to move away to be in a state of minimum repulsion for a stable covalent bond.
The VSEPR theory proposes that both bonded and lone electron pairs repel each other because they have the same type (negative) of charge. These electron pairs repel in a way so that they can minimise the repulsion and achieve stability to form a bond.
The VSEPR theory predicts the molecular structure based on the presence of electron pairs and their nature. Such as, through VSEPR theory we can explain has a triangular shape, which is the result of one lone pair in the molecule.
Key Principles of VSEPR Theory
VSEPR theory predicts the molecular geometry based on certain principles. Read those important principles below:
Electron Pairs Repulsion:
The electron pairs in the valence shell of a central atom repel each other because electron pairs have negative charge, which leads to electrostatic repulsion.
These electron pairs repel each other to be as far apart as possible so that the repulsion is minimized (Since the repulsive electrostatic force is inversely proportional to the displacement between pairs). This condition leads to the formation of a stable covalent bond.
Molecule Categories:
There are two types of electron pairs around the core/central atom: lone pair and bond pair. A bond pair is a pair of electrons shared between two atoms to form a covalent bond. A lone pair is a pair of electrons that does not participate in bonding.
There are two types of molecules based on electron pairs.
- Molecules in which the central atom has no lone pair. These molecules are denoted by AB type.
- Molecules in which the central atom has one or more lone pairs. These molecules are denoted by the ABE type.
Lone Pairs vs Bond Pairs Repulsion:
There is repulsion between all electron pairs present around the central atom. However, the amount of repulsive force is not at all equal. They do not repel in the same amount. The order of repulsion is given below:
(Lone Pair - Lone Pair (LP-LP) > Lone Pair - Bond Pair (LP-BP) > Bond Pair - Bond Pair (BP-BP)
This hierarchy is crucial to understanding the changes in bond angle, even though the number of electron pairs is the same.
Steric Number:
The steric number is an important tool measure bond order and understand the molecular structure. You can check the simple table below, predicting the molecular geometry based on the steric number.
| Steric Number | Molecular Shape |
|---|---|
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |
Postulates of the VSEPR Theory
The main postulates of VSEPR theory are as follows:
- Molecular geometry (shape) depends on the number of valence shell electron pairs (Bond pairs and lone pairs) around the central atom.
- The electron pairs in the valence shell, whether bonded or lone pair, repel each other since all electron pairs are negatively charged.
- The electron pairs align themselves to occupy such positions in space that minimise electrostatic repulsion, maximising distance between them.
- The electron pairs occupy such positions at maximum distance from one another on the spherical surface (valence shell).
- More than one (two or three) electron pairs of a multiple bond (double or triple) between two atoms are treated as a single super electron pair.
- The repulsion between two lone pairs is maximum, and between two bond pairs is minimum.
- VSEPR Theory can be used to predict the molecular shape if two or more resonance structures representing single molecule
The VSEPR theory follows an important electronic configuration for considering the covalent bond formation, including the Pauli exclusion principle and the Aufbau principle.
Steric (VSEP) Number
The steric number of a central atom is a key concept used to predict the three-dimensional molecular shape and the hybridization of orbitals. The steric number is mainly a number consisting of information about bonded and lone pairs. A clear understanding of Lewis structure is important to find the number of bonded atoms and lone pairs on the central atom.
The steric number is defined as the sum of the number of atoms bonded to the central atom and the number of lone pairs on the central atom.
The formula for the steric number is given below:
- Steric Number (SN) = (Number of atoms bonded to the central atom) + (Number of lone pairs on the central atom)
Or
- Steric Number (SN) = ½ [ (Number of valence electrons of the central atom) + (Number of monovalent atoms attached) – (Cationic charge) + (Anionic charge) ]
You can check the table below explaining the simple relation between molecular shape and steric number.
Table- Molecules with No Lone Pairs on the Central Atom
| Molecular Shape | Hybridization | Example | |
|---|---|---|---|
| 2 | Linear | sp | BeCl₂ |
| 3 | Trigonal Planar | sp² | BF₃ |
| 4 | Tetrahedral | sp³ | CH₄ |
| 5 | Trigonal Bipyramidal | sp³d | PCl₅ |
| 6 | Octahedral | sp³d² | SF₆ |
Table- Molecules with one or more Lone Pairs on the Central Atom
| Bonding Pairs | Lone Pairs | Molecular Shape | Hybridization | Example | |
|---|---|---|---|---|---|
| 3 | 2 | 1 | Bent (or Angular) | sp² | SO₂ |
| 4 | 3 | 1 | Trigonal Pyramidal | sp³ | NH₃ |
| 4 | 2 | 2 | Bent (or Angular) | sp³ | H₂O |
| 5 | 4 | 1 | See-Saw | sp³d | SF₄ |
| 5 | 3 | 2 | T-shaped | sp³d | ClF₃ |
| 5 | 2 | 3 | Linear | sp³d | XeF₂ |
| 6 | 5 | 1 | Square Pyramidal | sp³d² | BrF₅ |
| 6 | 4 | 2 | Square Planar | sp³d² | XeF₄ |
How to Determine Molecular Shape
You can use the following steps to find out the molecular structure of the molecule.
Step 1: Draw the Lewis Structure
Lewis structure is the arrangement of atoms and the distribution of valence electrons, including both bonding pairs and lone pairs. after drawing the Lewis structure, choose the least electronegative atom as the central atom.
Step 2: Stric Number: Count Electron Groups Around the Central Atom
Calculate the steric number by counting all the electron groups around the central atom. The electron groups are single bond, double bond triple bond, and lone pair of electrons present. You can use the above formula as well.
Step 3: Determine the Electron Geometry
Based on the calculated steric number, you can identify the molecular structure. We have provided a simple table above to identify the electron geometry of the molecule.Step 4: Determine the Molecular Shape (Molecular Geometry)
After identifying the electron geometry of the molecule, you need to understand the type of molecule.
- If there are no lone pairs present around the central atom
- If there are lone pairs present around the central atom.
You can now identify the molecular shape based on the type of molecule. Let's understand with examples.
Methane (CH₄)
1. Draw the Lewis Structure: Methane Lewis structure has 4 valence electrons around the central carbon atom bonded by single covalent bonds.
2. Count Electron Groups: Using the steric number formula: SN = 4 (Covalent bonds) + 0 (zero lone pairs) = 4
3. Determine the Electron Geometry: As per the above table, you can see, the steric number 4 molecules have a tetrahedral electron geometry.
4. Determine the Molecular Shape: Since there are no lone pairs on the central carbon atom, the molecular shape will be the same as electron geometry.
So the molecular shape: Tetrahedral
Molecular Geometries and Examples
We have read above about the molecular structure identification. You can check the below given diagrams below to understand how bonded and lone pairs align themselves in different molecules. Based on the steric number, we know how many bonded atoms, bonded pairs, and lone pairs are taking part in the determination of molecular structure.
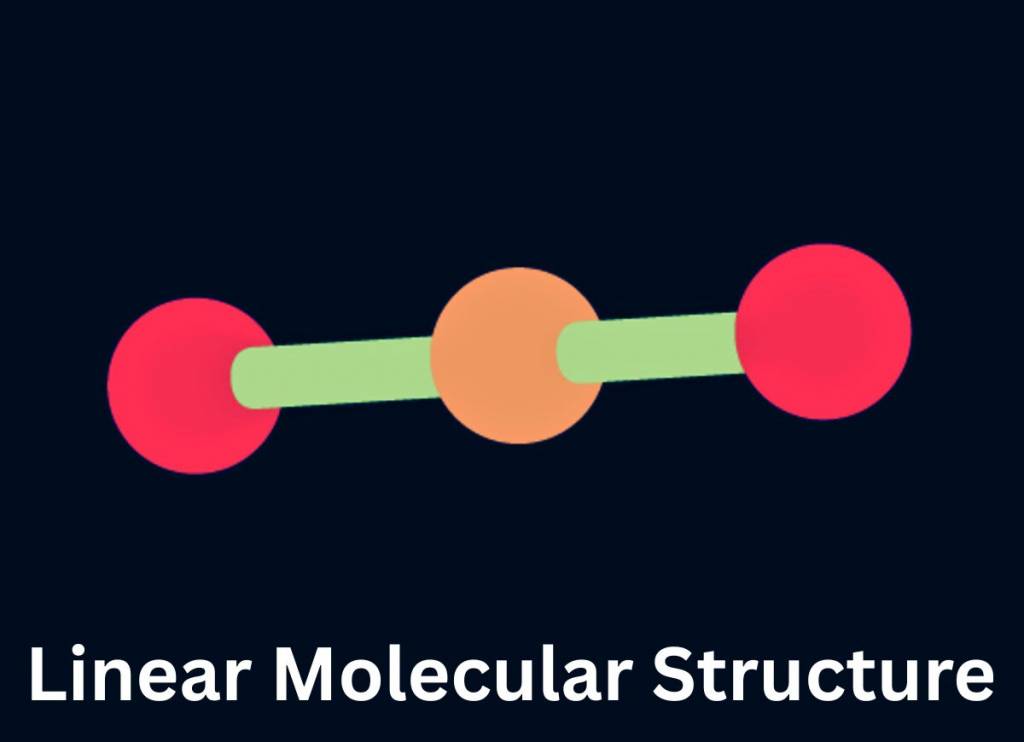
Linear Molecular Geometry (AB₂)
In this molecular geometry, two electron groups, including bonded pairs, lone pairs and bonded atoms align themselves in a straight line that stabilizes the molecule for the formation of a covalent bond with the central atom.
For example, Beryllium Chloride ( ) has a central atom (Beryllium) and two bonded atoms (Chlorine) with no lone pair electrons. The beryllium atom forms two single covalent bonds with both of the chlorine atoms. As per the VSEPR theory, each single bond can be considered as an electron domain. These electron domains arrange themselves to minimize repulsion. This process gives rise to a linear molecular structure.
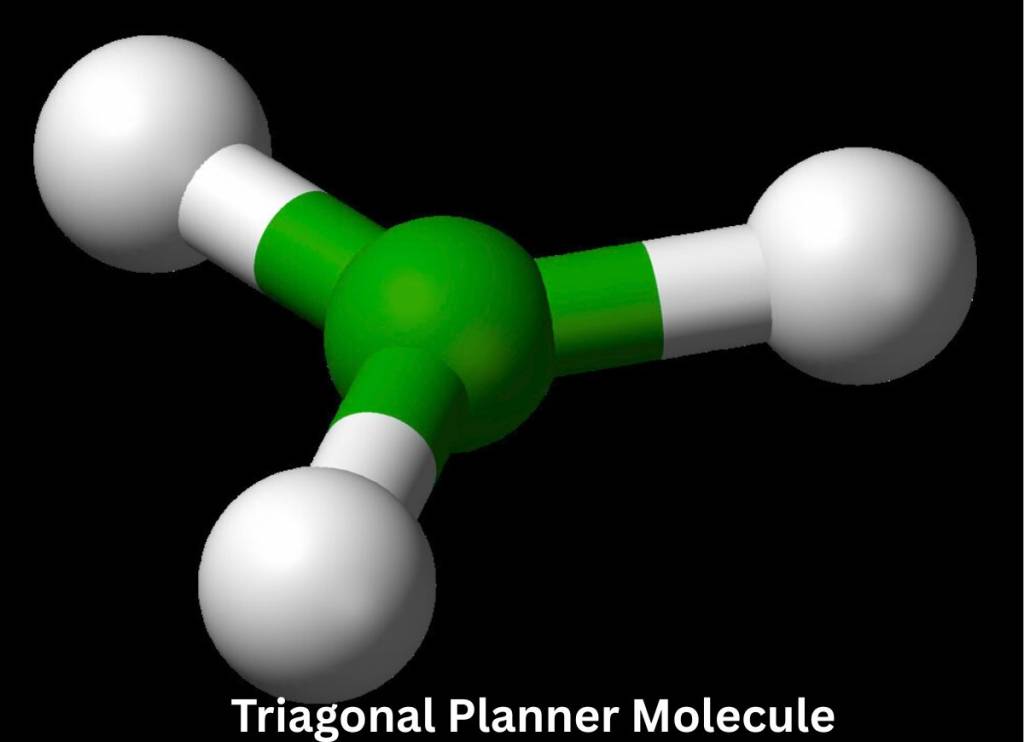
Triagonal Planar Molecular Geometry (AB₃)
This type of molecule includes three bonded pairs and no lone pairs, which leads to an equal amount of repulsion between them. These electron pairs align themselves in a triangular plane, at 120 degrees. Below is the 3D diagram of the Triangular Planner Molecule.
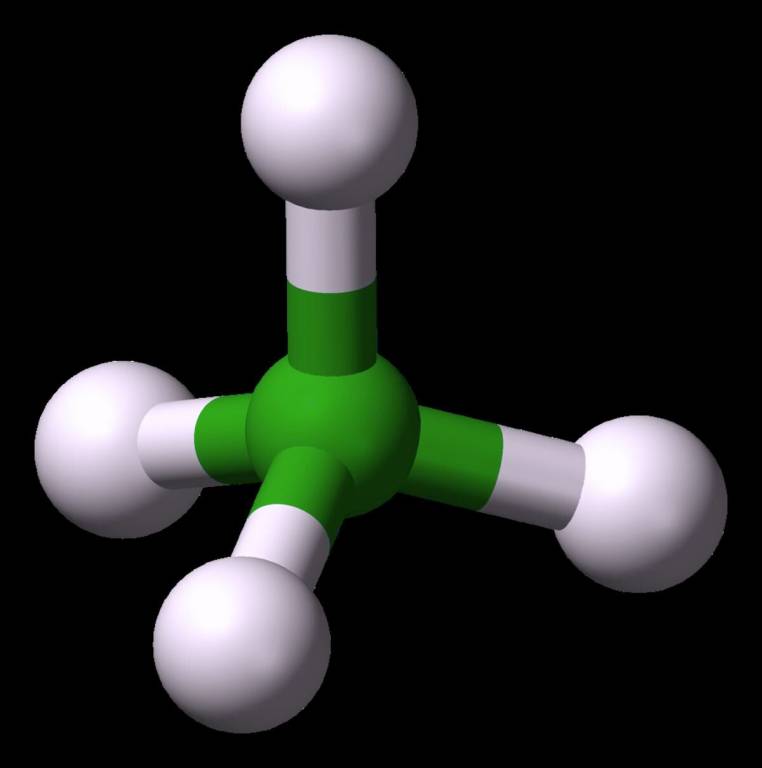
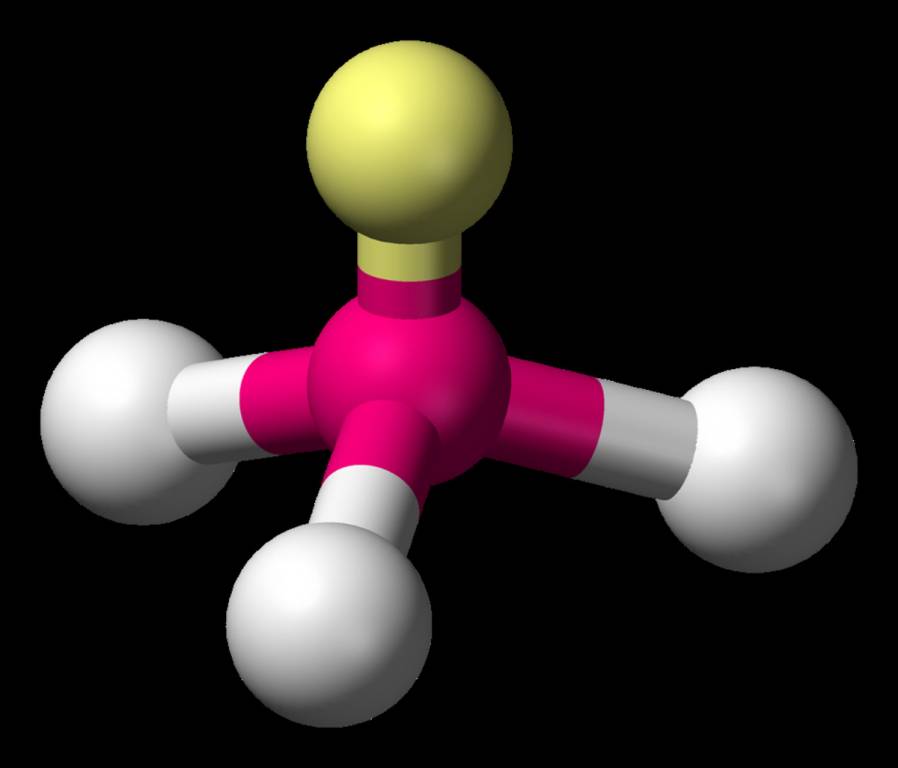
Tetrahedral Molecular Geometry (AB₄)
Ammonia is a prime example of tetrahedral molecular geometry. This type of molecule has 4 bonding pairs, 0 lone pairs, and they align themselves at a bond angle. These molecules shows hybridization properties. Check the diagram below.
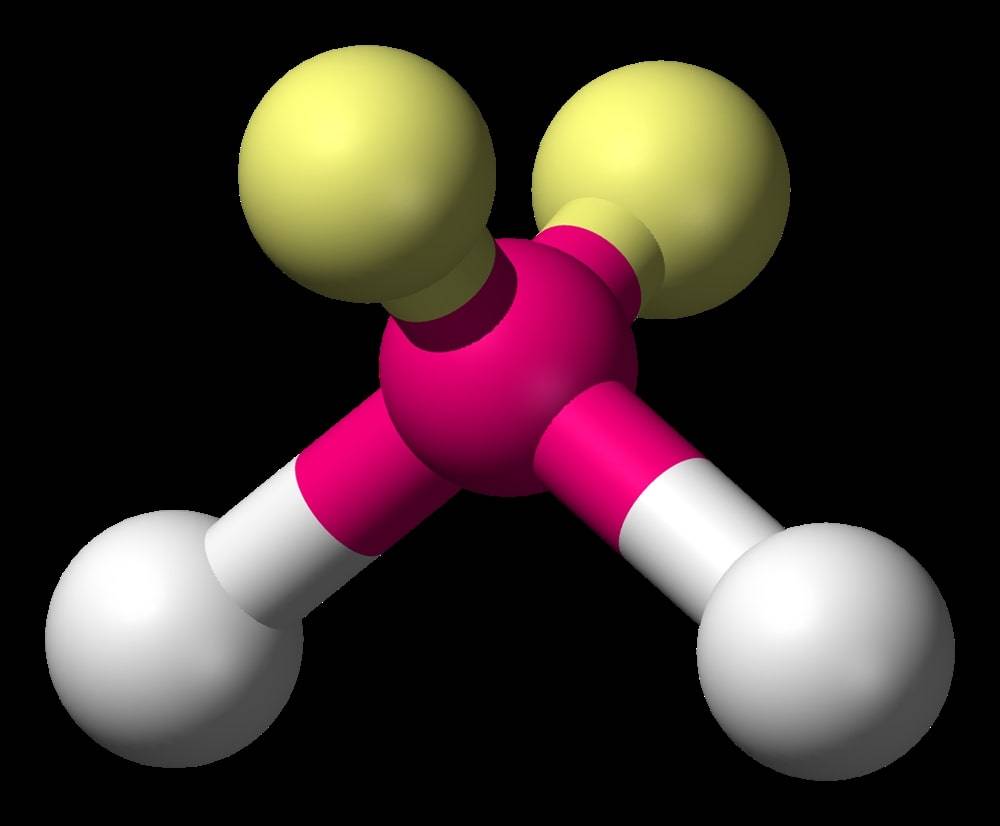
Bent or Angular Molecular Structure
Whenever a central atom has two bond pairs and two lone pairs of electrons, it leads to bending because of uneven repulsion and attraction between bonded and lone pairs. It gives rise to bent or angular molecular geometry.
Trigonal Bipyramidal Molecular Geometry:
This type of molecule has five bonding pairs and zero lone pairs around the central atom. Each of them make with the equatorial plane and with the axial plane. These molecules show hybridization.
Octahedral Molecular Geometry:
These molecules have six bonding pairs and zero lone pairs; all the electron groups share bond angles with each other, making a hybridization.
Factors Influencing Bond Angles
There are several factors that affect the bond angle. You can check the factors and how they affect the bond angle.
- Lone Pairs: The presence of lone pairs creates stronger repulsion, the stronger repulsion bends the bonds and reduces bond angles.
- Electronegativity: The higher the electronegativity of the central atom, the stronger it pulls bonding pairs closer. So high electronegativity reduces angles between bonds.
- Atomic Size: The atomic size of the central atom also affects the bond angles. Large central atoms have weak orbital overlap, which reduces bond angles. As in the case of vs .
- Multiple Bonds: The higher the bond order (Double or triple bonds ), the greater repulsion they exert on each other.
You can read about the effects on the bond angle and other important bond parameters due to molecular geometry.
Limitations of VSEPR Theory
Applications of VSEPR Theory
Study Materials for CBSE Class 11
NCERT Class 11 Chemistry Chapter-wise Notes
Commonly asked questions
What are the 5 postulates of VSEPR theory?
VSEPR theory predicts the shape of molecule based on postulate (assmptions). here are the important postulates as per the NCERT textbooks.
- The molecular geometry shape depends upon the number of valence shell electron pairs (bonded and non-bonded) around the central atom.
- The electron pairs (bonded and lone pairs) in the valence shell repel each other since their electron clouds are negatively charged.
- These electron pairs arrange themselves to minimize repulsion so that the molecule attains a stable structure with minimum energy.
- All electron pair repulsion doesn't repel each other equally, Electron pairs follows this order:
Lone pair–Lone pair > Lone pair–Bond pair > Bond pair–Bond pair - VSEPR theory only considers the valence shell electron pairs surrounding the central atom to predict the shape of the molecule.
What is VSEPR theory in class 11th Chemistry?
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is an algorithm developed to predict the molecular geometry of the compounds. The VSEPR theory predict the molecular shape based on the repulsion between electron pairs (bonding and lone) around the central atom. As per the NCERT Textbooks:
“According to this theory, the shape of a molecule depends upon the number of valence shell electron pairs around the central atom. Electron pairs repel each other and try to remain as far apart as possible to minimise repulsion, thus determining the geometry of the molecule.”
You can use this theory in primarily explaining the molecular structure of the chemical compounds with covalent bonding. You can read complete details related to the VSEPR Theory through our page.
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Molecules usually form chemical bond through either sharing or through rtransfering the electrons. During covalent bonding the electron pairs shared between atoms to form covalent bond are called shared pair or bond pair. At the same time, the electron pair which is not involved in sharing is called lone pair of electrons.
For example: CH4 has 4 bond pairs but H2O has 2 bond pairs and 2 lone pairs.

Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics