You must have seen how rain forms tiny spherical droplets on leaves, or a needle floats on water. In physics, there is a term that helps us learn the mechanism behind such formation. We call it surface tension.
Surface tension is a key concept in the chapter on Mechanical Properties of Fluids. When practising the Class 11 NCERT solutions for Chapter 9, you will find both numerical and theoretical exercises in it.
- What is Surface Tension?
- Working Principle of Surface Tension
- Examples of Surface Tension
- Causes of Surface Tension
- Factors affecting Surface Tension
What is Surface Tension?
Surface tension is the tendency of a liquid's surface to act like an elastic sheet.
Importance of Learning Surface Tension
- Surface tension relates to other chapter topics, such as Bernoulli’s Principle and Viscosity.
- Once you figure out how all these interrelated concepts of fluid dynamics work together, you not only score confidently in your annuals but also in engineering and medical entrances.
Working Principle of Surface Tension
Let’s examine why surface tension occurs on a liquid surface.
You see, molecules in a liquid attract each other through a force that scientists call cohesion. You might even remember this term from earlier science classes, or one you’ll explore further when studying biology in 11th class.
Because of cohesion, there are two scenarios to think about.
- Molecules deep inside the liquid pull on each other equally from all neighbouring sides. Here, the net force remains the same.
- But, visualise a situation or space where the water meets the air, much like the surface of a lake or sea. There are no water molecules directly above it. It cannot have any significant upward cohesive pull as compared to the sides and below. This creates an imbalance of forces. The forces on the sides and below still have an inward pull, which people term as the net inward force on the surface molecules. That very inward pull ‘tightens’ the surface, making it behave like an elastic sheet.
Examples of Surface Tension
1. A Drop of Liquid
Whether walking through the rain, spilling the morning coffee, or putting an eye drop, we come across several liquids splashing off the solid surfaces.
Most of the time, such events go unnoticed, but if one pays a significant amount of attention, they would notice that the basic physics that governs the dynamics of liquid droplets is extremely rich of complexities.
The hydrodynamics of a liquid drop under free fall is fascinating. It is easy to see that the drop seems to have a "skin" holding it into a sort of sphere.
The lowest energy state for this drop occurs when the maximum number of water molecules are surrounded by other water molecules without falling apart, meaning that the drop should have the minimum possible surface area, which is a sphere.
The effect of gravity distorts this ideal sphere into a nearly-oval shape that we see. In the absence of other forces, including gravity, drops of virtually all liquids would be approximately spherical.
2. Soaps and Detergents
Soaps and detergents seem like simple things that we can find in our bathrooms, laundry area, or cleaning supplies. Just splash some water on your face, apply soap, rinse again, and all the dirt is gone, right?
Still, the chemistry behind this phenomenon is not that simple. Soaps and detergents are quite tricky chemicals, and they work in a really interesting way.
Water molecules prefer to stick together through intermolecular forces. Soaps and detergents help the cleaning by lowering the surface tension of the water so that it can more readily soak into pores and soiled areas.
The soap molecules are composed of long chains of carbon and hydrogen atoms along with other ionic molecules. At one end of the chain is a configuration of atoms that likes to be in water (hydrophilic).
The other end, however, shuns water (hydrophobic) but attaches easily to grease. In washing, the "grease-loving" end of the soap molecule attaches itself to the oil or fat present on the stain, letting water flow underneath. The particles of stain become loose and surrounded by soap molecules, to be carried off by a flood of water.
3. Water Striders
Water striders are a family of insects that are capable of walking on water. They are also known by other common names such as water skaters, water scooters, water bugs, pond skaters, water skippers, Jesus bugs, or water skimmers.
They use the surface tension of water to their advantage. Combined with their long, thin, and hydrophobic legs, which allow the weight of the water strider body to be distributed over a large surface area, the surface tension of water provides them with necessary shielding from the force of gravity that can cause them to sink.
Nevertheless, if by any natural cause such as waves, a water strider submerges, the tiny hairline presents on its body traps enough air to provide buoyancy to bring the water strider back to the surface again, while also providing air bubbles to breathe underwater.
Causes of Surface Tension
Due to the cohesive forces, a molecule located away from the surface is pulled equally in every direction by neighbouring liquid molecules, resulting in a net force of zero.
The molecules at the surface do not have the same molecules on all sides of them and therefore are pulled inward. This creates some internal pressure and forces liquid surfaces to contract to the minimum area.
There is also a tension parallel to the surface at the liquid-air interface which will resist an external force, due to the cohesive nature of water molecules.
The forces of attraction acting between molecules of the same type are called cohesive forces, while those acting between molecules of different types are called adhesive forces.
The balance between the cohesion of the liquid and its adhesion to the material of the container determines the degree of wetting, the contact angle and the shape of meniscus.
When cohesion dominates (specifically, adhesion energy is less than half of cohesion energy) the wetting is low and the meniscus is convex at a vertical wall (as for mercury in a glass container).
On the other hand, when adhesion dominates (adhesion energy more than half of cohesion energy) the wetting is high and the similar meniscus is concave (as in water in a glass).
Surface tension is responsible for the shape of liquid droplets. Although easily deformed, droplets of water tend to be pulled into a spherical shape by the imbalance in cohesive forces of the surface layer. In the absence of other forces, drops of virtually all liquids would be approximately spherical.
The spherical shape minimizes the necessary "wall tension" of the surface layer according to Laplace's law.
Another way to view surface tension is in terms of energy. A molecule in contact with a neighbour is in a lower state of energy than if it were alone.
The interior molecules have as many neighbours as they can possibly have, but the boundary molecules are missing neighbours (compared to interior molecules) and therefore have a higher energy.
For the liquid to minimize its energy state, the number of higher energy boundary molecules must be minimized. The minimized number of boundary molecules results in a minimal surface area.
As a result of surface area minimization, a surface will assume the smoothest shape it can (mathematical proof that "smooth" shapes minimize surface area relies on use of the Euler-Lagrange equation). Since any curvature in the surface shape results in greater area, a higher energy will also result
Factors affecting Surface Tension
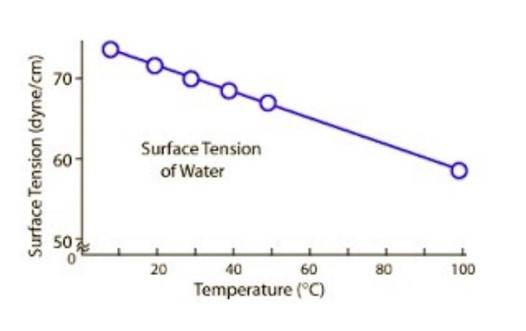
TEMPERATURE:
1. It is one of the main factor affecting surface tension.
2. When the temperature increases, the molecular thermal activity increases.
3. The increase in molecular thermal activity causes decrease in cohesive interaction. This causes decrease in surface tension.
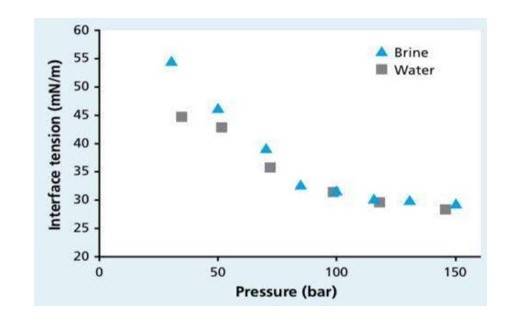
PRESSURE:
1. It is the other factor affecting the surface tension.
2. In general, dissolution of gas in liquid increases with the increasing pressure, decreasing the surface tension.
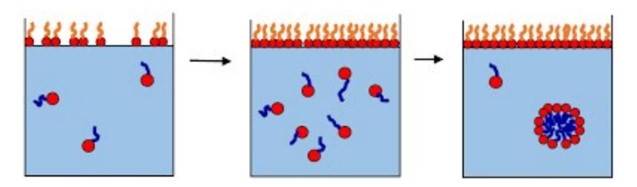
SURFACTANTS:
1. It is also called surface-active agent, substance such as a detergent that, when added to a liquid, reduces its surface tension, thereby increasing its spreading and wetting properties.
2. The surface-active molecule must be partly hydrophilic (water-soluble) and partly lipophilic (soluble in lipids, or oils). It concentrates at the interfaces between bodies or droplets of water and those of oil, or lipids, to act as an emulsifying agent, or foaming agent.
3. When surfactants are dissolved in water, they migrate to the surface/interfaces and orientates themselves at the surface with the hydrophilic parts in water and hydrophobic parts in air.
4. They absorb at air-water interface and forms a mono layer.
5. The surface tension is reduced as some of the water molecules are replaced by the surfactant molecules and the interaction force between the surfactant and water is less than between two water molecules.
6. At higher surfactant concentrations they form micelles with head facing the water and tail facing inside.
7. The concentration at micelles starts to form is called critical micelles concentration(CMC).
After CMC increase in concentration only adds to formation of more / bigger micelles
Physical units:
Surface tension, represented by the symbol (alternatively or ), is measured in force per unit length. Its SI unit is newton per meter but the cgs unit of dyne per centimetre is also used. For example,
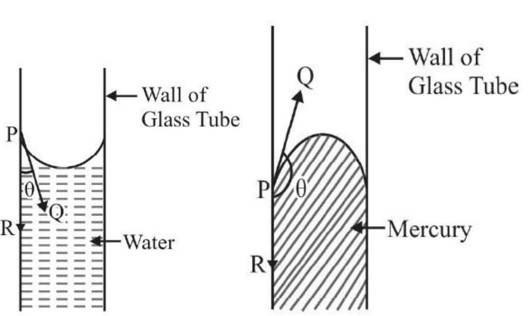
Contact angles:
The surface of any liquid is an interface between that liquid and some other medium. The top surface of a pond, for example, is an interface between the pond water and the air.
Surface tension, then, is not a property of the liquid alone, but a property of the liquid's interface with another medium. If a liquid is in a container, then besides the liquid/air interface at its top surface, there is also an interface between the liquid and the walls of the container.
(a) (b)
The surface tension between the liquid and air is usually different (greater) than its surface tension with the walls of a container. And where the two surfaces meet, their geometry must be such that all forces balance.
Where the two surfaces meet, they form a contact angle,
, which is the angle the tangent to the surface makes with the solid surface.
Note that the angle is measured through the liquid, as shown in the diagrams above. The diagram to the right shows two examples. Tension forces are shown for the liquidair interface, the liquid-solid interface, and the solid-air interface.
The example on the left is where the difference between the liquid-solid and solid-air surface tension, , is less than the liquid-air surface tension, , but is nevertheless positive, that is
In the diagram, both the vertical and horizontal forces must cancel exactly at the contact point, known as equilibrium. The horizontal component of is cancelled by the adhesive force,
Since the forces are in direct proportion to their respective surface tensions, we also have:
Where
- is the liquid-solid surface tension,
- is the liquid-air surface tension,
- is the solid-air surface tension,
- is the contact angle, where a concave meniscus has contact angle less than and a convex meniscus has contact angle of greater than
Physics Mechanical Properties of Fluids Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion