- What is First Law of Thermodynamics?
- NCERT Definition of First Law of Thermodynamics with Formula
- Specific Heat Capacity
- Sign Convention
- Important Points of the First Law of Thermodynamics
- Limitations of First Law
What is First Law of Thermodynamics?
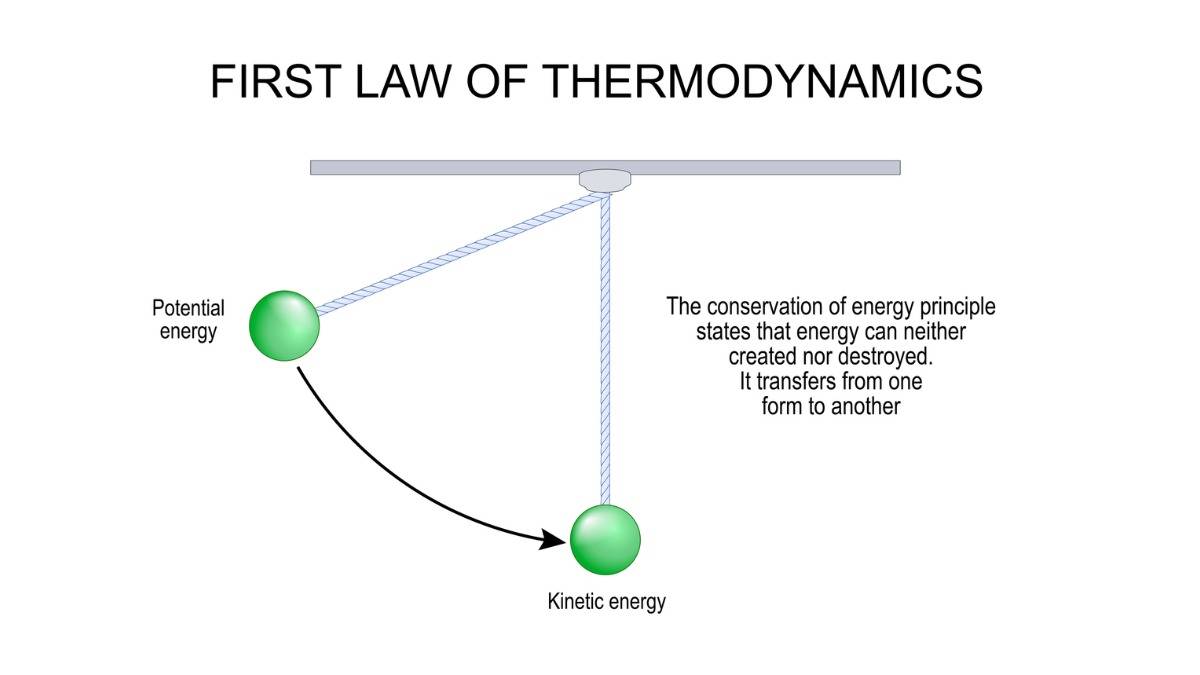
The First Law of Thermodynamics is an energy conservation rule that we use for understanding heat and work.
To put simply, the 1st Law of Thermodynamics states that energy is neither created nor destroyed. Energy can only change its form.
The basic First Law of Thermodynamics formula is
is the change of internal energy
is the heat you add to the system
is the work done by the system
Importance of the 1st Law of Thermodynamics
- The First Law of Thermodynamics is a part of engineering entrance exams in India. Based on previous years' JEE Mains, you can expect a question or two on this. You may be asked to calculate internal energy changes under different thermodynamic processes.
- There are common errors you could avoid when answering the First Law of Thermodynamics-based questions in entrance exams. Confusions, such as 'work done by' and 'work done on' can wholly let you misinterpret problem statements. Once you are clear with this concept with formula and its applications, you will be able to confidently answer and secure good marks.
NCERT Definition of First Law of Thermodynamics with Formula
Let's explain the First Law of Thermodynamics definition with formula.
Heat that the surroundings supply to the system
Work done on the surroundings by the system
Internal energy changing in the system
So, the general principle of conservation of energy will imply that
……(1)
It means that supplying the energy to the system enters partially and increases its internal energy . While the rest goes to work on the environment .
This is the First Law of Thermodynamics equation.
It's a general law of conservation of energy that applies to any system where considering energy transfer from or to the surroundings.
Now, let's put Eq. (1) in an alternative form
……(2)
Here, the system could go from an initial to the final state in different ways.
For an example's sake,
To change the gas state from to , we can initially change the gas volume to . Note that the pressure is kept constant.
Meaning, we can first reach this state . After that, we change the gas pressure from to .This is to keep the volume constant, so that gas reaches ( ).
Conversely, we could initially keep the volume constant. And also maintain a constant pressure.
, being a state variable, will depend solely on the first and final states. It will not depend on the path the gas had taken to go from one state to another.
But, usually, and will be dependent on the path taken to reach between the initial and final states.
From the second equation (Eq. 2) of the First Law of Thermodynamics, the combination , is however, path independent. Now this highlights that when a system is going through a process where
we get
(If you had learnt about isothermal expansion of an ideal gas, you will know this).
So here, the heat that is supplied to the system is completely used by it when doing work on the environment.
Now, in a system with a cylinder and a movable piston, the gas does work as it moves the piston.
Also, we know, force is pressure times area. While area times displacement is volume. So the work done by the system against a pressure that constant, i.e.,
is
Here, is the change in gas volume. Thus, for this case, Eq. (1) gives
As an application of the second equation, Eq. (2), consider the change in internal energy for 1 g of water when we go from its liquid to vapour phase.
is the measured latent heat of water. So, for 1 g of water .
When considering atmospheric pressure, 1 g of water has a volume
in liquid phase and
in vapour phase.
Therefore,
Equation (2) then gives
We see that most of the heat goes to increase the internal energy of water in transition from the liquid to the vapour phase.
Specific Heat Capacity
Let's consider the temperature change from to when a heat amount is supplied to a substance.
We define heat capacity of a substance to be
We expect
and heat capacity
to be proportional to the substance's mass. Further, it could also depend on the temperature.
The heat needed to raise the temperature by one degree can vary depending on the temperature.
To define a constant characteristic of the substance and independent of its amount, we divide by the mass of the substance in kg :
is the substance's specific heat capacity. It depends on the nature of the substance and its temperature. This is dependent on the substance's nature and temperature. The specific heat capacity's unit is .
When we specify the substance amount in moles (instead of mass in kg ), it is easier to define or show heat capacity per mole of the substance by
is the substance's molar specific heat capacity. Similar to s , C is independent of the substance amount. C depends on the nature of the substance. Like is independent of the amount of substance. Overall, is dependent on the substance's nature, its temperature and the conditions under which heat is supplied
The unit of
is
.
We will observe later in the study of specific heat capacity of gases, there would be extra conditions to define
or
. The purpose of defining
is that we can make simple predictions with molar specific heat capacities.
The table below highlights measured specific and molar heat capacities of solids at atmospheric pressure and ordinary room temperature.
In coming chapters of the physics NCERT textbook, we will see that predictions of specific heats of gases usually agree with experiment.
We could apply the same equipartition of energy law and easily predict the solid's molar specific heat capacities.
Suppose, there's a solid of atoms. Each of them vibrates about its mean position. An oscillator in one dimension has average energy of . In three dimensions, the average energy is . For a mole of a solid, the total energy is
Now, at constant pressure, , since for a solid is negligible. Therefore,
Table 1 Specific and molar heat capacities of some solids at room temperature and atmospheric pressure
| Substance |
Specific |
Molar specific heat |
| Aluminium |
900.0 |
24.4 |
| Carbon |
506.5 |
6.1 |
| Copper |
386.4 |
24.5 |
| Lead |
127.7 |
26.5 |
| Silver |
236.1 |
25.5 |
| Tungsten |
134.4 |
24.9 |
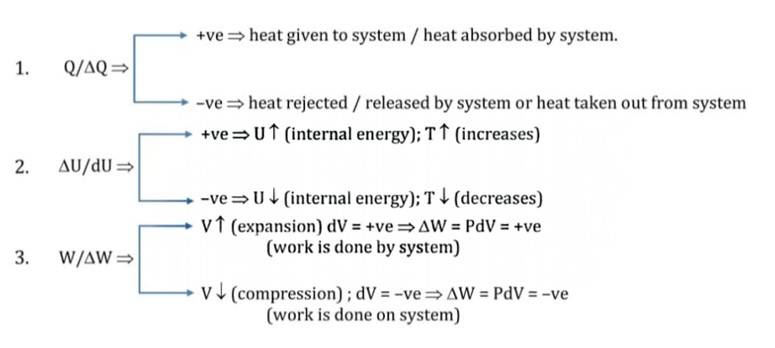
Sign Convention
Important Points of the First Law of Thermodynamics
- We can apply the First Law of Thermodynamics to many of nature's processes. This law holds true for all three states of matter: solid, liquid, and gas.
- It's a well-known fact that thermodynamic force is non-conservative. The First Law of Thermodynamics is based on the law of conservation of energy.
- The First Law of Thermodynamics is about internal energy. What this is means that it's the energy stored within a system. That plays an important role in how heat is managed and utilised within that system.
- Make sure that , and dW share the same units for a seamless equation. (either in units of work or in units of heat).
- dU is a characteristic of the state of a system; it may be any type of internal energy-translational kinetic energy, vibrational, rotational kinetic energy, binding energy, etc.
Limitations of First Law
First law is a statement of conservation of energy principle. Satisfaction of first law alone does not ensure that the process will actually take place.
Examples:
- A cup of hot coffee left in a cooler room eventually cools off. The reverse of this process- coffee getting hotter as a result of heat transfer from a cooler room does not take place.
- Consider heating of a room by passage of electric current through an electric resistor. Transferring of heat from room will not cause electrical energy to be generated through the wire.
- Consider a paddle-wheel mechanism operated by fall of mass. Potential energy of mass decreases and internal energy of the fluid increases. Reverse process does not happen, although this would not violate first law.
- Water flows down hill where by potential energy is converted into K.E. Reverse of this process does not occur in nature.
Physics Thermodynamics Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test