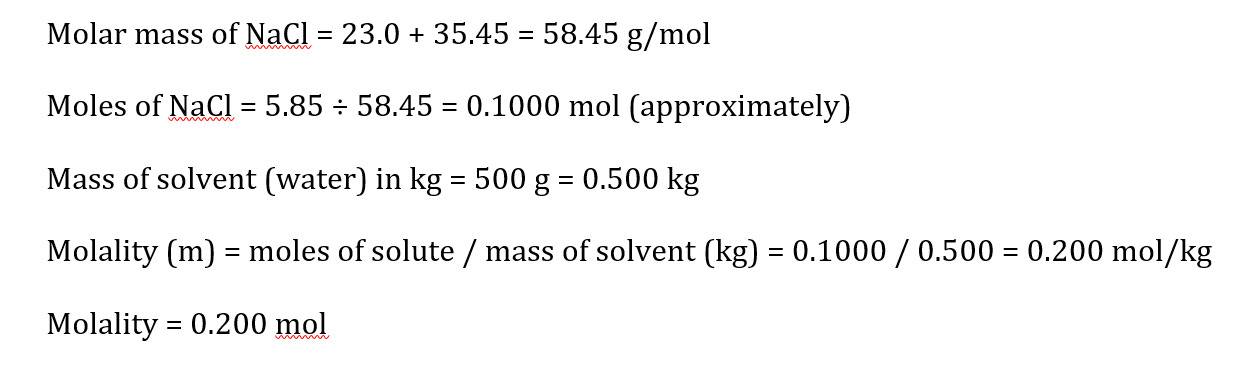Our subject matter experts as Shiksha, have created conceptually rich and quality NCERT Solutions for Class 12 Chemistry Chapter 1. These include intext Class 12 Solutions questions and answers in a descriptive manner. We have provided a step-by-step explanation according to the Class 12 CBSE Board exam.
Students can also download and utilise the PDF version of the complete solutions. The NCERT Class 12 Chemistry Chapter 2 Solutions PDF link is given below. It includes intext question solutions of the Class 12 Chemistry Solution chapter.
Class 12 Chapter 1 Solutions include various important topics such as Types of Solutions, Concentration, Molarity, Molality, ppm, Solubility, Henry's Law, Raoult's Law, Colligative Properties and Osmosis. These topics holds immense importance for board exams, competitive exams such as JEE Mains and others.
While preparing these NCERT Solutions, we at Shiksha focus on providing error-free, accurate, in-depth and trustworthy study material. You can completely rely on Shiksha's Class 12 Chemistry NCERT Solutions to prepare for board and other state board exams (UP Board, MP Board, etc).
Along with solved NCERT exercises, you can access all topics, concepts, important formula sheet, important questions and quick revision notes through this page. Explore the questions and answers below.
- Download Chemistry Class 12 Solutions NCERT Solution PDF for Free
- NCERT Solutions for Class 12 Chemistry Chapter 1 Exercises
- Complete Class 12 Chemistry Solutions Study Material
- Class 12 Chemistry Chapter 1 Solutions Important Topics
- Chemistry Class 12 Chapter 1 Solutions Important Formula Sheet
- Class 12 Chemistry Solutions: Weightage in CBSE , JEE and NEET
- Important Questions of Solutions Class 12 Chemistry
- Class 12 Chemistry Study Material
Download Chemistry Class 12 Solutions NCERT Solution PDF for Free
Many students don't have a steady internet connection. Also, some of them want to use the internet as less as possible to avoid distractions. We offer all these Class 12 Chemistry Chapter 1 Solutions intext question and answers in PDF format. You can download the PDF for free and study anytime and anywhere you want. Click on the link below:
Download Here: NCERT Solutions for Class 12 Chemistry Solutions
NCERT Solutions for Class 12 Chemistry Chapter 1 Exercises
Students can find the solutions for the Chemistry Class 12 Solution Chapter 2 exercises below. The solutions are prepared by the renowned teachers. Solving these questions, students can prepare themselves for the CBSE Class 12 board exam. In case of any confusion, seek the help of the class teacher to understand complex problems easily.
| Exercise Q 2.1 Define the term solution. How many types of solutions are formed? Write briefly about each type with an example |
||||||||||||||||||||||||||||||||||||||||
| A 2.1 A solution is a homogeneous mixture of two or more than two substances on molecular level whose composition can vary within certain limits. The part or component of the mixture present in a lesser amount is called the SOLUTE and the one present in larger amount is called the SOLVENT. For eg- small amount of salt [solute] dissolved in water [solvent]. There are nine types of solutions formed. They are:
Out of these nine types solution, solid in liquid, liquid in liquid & gas in liquid are very common. When the components of the solution are mixed, the resulting solution may exist in any of the three possible states of matter that is solid, liquid or gaseous. They are:
|
| Q 2.2 Give an example of a solid solution in which the solute is a gas. |
| A 2.2 As the name signifies, a solid solution is one in which solvent is solid.So considering this aspect absorption of hydrogen over platinum or palladium is an example of such solution. Platinum or palladium is used as a catalyst in hydrogenation processes. |
| Q 2.3 Define the following terms: (i) Mole fraction (ii) Molality (iii) Molarity (iv) Mass percentage |
| A 2.3 (1) Mole fraction - The mole fraction of a particular component in a solution is the ratio of the number of moles of that component to the total number of moles of all the components present in the solution. Mathematically, Mole Fraction of component = Number of moles of given component / Total number of moles in solution Mole Fraction is independent of temperature.
Molality actually represents the concentration of solution in mol / kg. Mathematically, Molality = Number of moles of solute/ Mass of solvent in kg It is represented by m.
Molarity actually represents the concentration of a solution in mol / L. Mathematically, Molarity = Number of moles of solute/ Volume of solution in litres
For example, 10% [by mass] urea solution means that 10 g of urea are present in 100 g of solution, the solvent being only 100-10 = 90 g. Mathematically, the mass percentage of a solute in a solution is given by: Mass Percentage of Solute = Mass of solute / Mass of solute + Mass of solvent X 100 Or Mass Percentage of Solute = Mass of solute / Mass of solution X 100 |
| Q 2.4 Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504 g mL^–1? |
| A 2.4 Given: Concentration of Nitric Acid, HNO3 = 68% Density of solution, d = 1.504 g/ml To find: Molarity, Mo Formula: Density, d = Mass (M) / volume (V) Molarity, Mo = Number of moles of solute/ Volume of solution in litres Solution: 68% of Nitric acid by mass in aqueous solution means that 68g [[68 × 100]/100] of Nitric acid present in 100g of solution. ⇒ Molecular mass of Nitric Acid, HNO3 = [1 × 1] + [1 × 14] + [16 × 3] = 63g ⇒ Number of moles of Nitric Acid = [68/63] = 1.079 moles ⇒ Given Density, d = 1.504 g/ml ⇒ Volume, v = [100/1.504] = 66.489 ml ⇒ Molarity, Mo = [1.079/66.489] × 1000 = 16.23 M Therefore the molarity of the sample is 16.24 M. |
Commonly asked questions
Q 2.41 Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25° C, assuming that it is completely dissociated.
Given-
Mass of K2SO4, w = 25 mg = 25 X 10-3 g,
Molar mass of K2SO4 = (39×2) + (32×1) + (16×4) = 174 g mol-1
Volume V = 2 liter
T = 250C + 273 = 298 K (add 273 to convert in Kelvin)
The reaction of dissociation of K2SO4 is written as,
K2SO4 → 2K + + SO42-
Number if ions produced = 2 + 1 = 3, hence vant Hoff’s factor, I = 3
Here, we use vant Hoff’s equation for dilute solutions, given as,
πV = inRT
where, n is the number of moles of solute, R is solution constant which is equal to the gas constant (0.082) and T is the absolute temperature (298 K).


Hence, the osmotic pressure of a solution is 5.27x10-3atm
Q 2.53 Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
Given,
Volume of water, V = 450 mL = 0.45 L
Temperature, T= (37 + 273)K = 310 K
1.0 g of polymer of molar mass 185,000
Number of moles of polymer, n = 1 / 185,000 mol
We know that,
Osmotic pressure? = nRT/V
= 1 X 8.314 X 103 X 310 / 185000 X 0.45
= 30.98 Pa
= 31 Pa (approx)
Q 2.19 A solution containing 30 g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further, 18 g of water is then added to the solution and the new vapour pressure becomes 2.9 kPa at 298 K. Calculate: (i) molar mass of the solute (ii) vapour pressure of water at 298 K.
Given: mass of solute = 30g
Let the molar mass of solute be x g and vapour pressure of pure water at 298k be P1 ?
Mass of water(solvent) = 90g
Molar mass of water = H2O = 1 × 2 + 16 = 18g
Moles of water = mass of water/molar mass
⇒ n = 90/18 moles
⇒ n = 5moles
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
x2 = (30/x) / (30/x) + 5
x2 = 30 / 30+5x
Vapour pressure of solution (p1) = 2.8kpa
Applying the formula:

According to second condition when we add 18g of water to solution vapour pressure becomes 2.9kpa
Moles of water = mass/molar mass
⇒ n = 90 + 18/18
⇒ n = 6moles
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
x2 = (30/x) / (30/x) + 6
x2 = 30 / 30+6x
Applying the formula:

Solving 1 and 2:
Dividing (2) by (1) we get


(i) molar mass of the solute = 78g
(ii) vapour pressure of water at 298 K = 537kpa
Q 2.52 Calculate the mass of ascorbic acid (Vitamin C, C6H8O6 ) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5°C. Kf = 3.9 K kg mol^-1.
Given
Mass of acetic acid, w1 = 75 g
Lowering of melting point? Tf = 1.5 K
Kf = 3.9 K kg/mol
Molar mass of ascorbic acid (C6H8O6), M2 6 * 12 + 8 * 1 + 6 * 16 = 176 g/mol
We know that,

= 5.08 g
Hence,
5.08 g of ascorbic acid is needed to be dissolved.
Q 2.26 If the density of some lake water is 1.25g mL^–1 and contains 92 g of Na+ ions per kg of water, calculate the molarity of Na+ ions in the lake.
Mass of ions = 92g
Molar mass of ions = Na+ = 23g (neglect the mass lost due to absence of a electron)
Moles of ions = mass of ions/molar mass
⇒ n = 92/23 moles
⇒ n = 4moles
Molality of solution = moles of solute/mass of solvent (in kg) Molality = 4/1 = 4M
Q 2.50 Vapour pressure of pure water at 298 K is 23.8 mm Hg. 50 g of urea (NH2CONH2 ) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Given,
Vapour pressure of water, PIo = 23.8 mm of Hg
Weight of water, w1 = 850 g
Weight of urea, w2 = 50 g
Molecular weight of water, M1 = 18 g/mol
Molecular weight of urea, M2 = 60 g/mol
n1 = w1/M1 = 850/18 = 47.22 mol
n2 = w2/M2 = 50/60 = 0.83 mol
We have to calculate vapour pressure of water in the solution p1
By using Raoult's therom,

PI = 23.4 mm of Hg Hence,
The vapour pressure of water in the solution is 23.4mm of Hg and its relative lowering is 0.0173.
Q 2.44 Calculate the molarity of each of the following solutions: (a) 30 g of Co(NO3)2. 6H2O in 4.3 L of solution (b) 30 mL of 0.5 M H2SO4 diluted to 500 mL.
Molarity = Moles of Solute / Volume of Solution in liter
(a) Given, In 4.3 L of solution there is 30 g of Co (NO3)2. 6H2O
Molar mass of Co (NO3)2.6H2O = (1 × 59 + 2 × (1 × 14 + 3 × 16) + 6 × 18)
= 291 g/mol.
∴ Moles = Given Mass / Molar Mass = 30/291 = 0.103 mol.
Now, Molarity = 0.103 mol / 4.3 L
= 0.023 M
(b) Given, 30 mL of 0.5 M H2SO4 diluted to500 mL.
In 1000 mL of 0.5 M H2SO4, number of moles present is 0.5 mol.
∴ In 30 mL of 0.5 M H2SO4, number of moles present = 30X 0.5 / 1000 mol.
= 0.015 mol.
∴ Molarity = 0.015 mol / 0.5L
= 0.03 M.
Q 2.23 Suggest the most important type of intermolecular attractive interaction in the following pairs. (i) n-hexane and n-octane (ii) I2 and CCl4 (iii) NaClO4 and water (iv) methanol and acetone (v) acetonitrile (CH3CN) and acetone (C3H6O)
(i) Both the compounds are non-polar and they do not attract each other because they do not form any polar ions. Vanderwaals forces of attraction will be dominant in between them as vanderwaals forces of attraction are not a result of any chemical or electronic bond.
(ii) now here both the compounds are non-polar because in I2 both the atoms are same so they have same electronegativity and hence there will be no displacement of electron cloud, it will be in the centre. In case of CCl4 molecule, it has tetrahedral shape so two Cl atoms will cancel the attraction effect from two opposite Cl atoms, hence molecule as a whole is non polar. Therefore they will also have vanderwaals forces of attraction.
(iii) NaClO4 is ionic in nature as Na, Cl and O all have different electronegativity and their shape is also not symmetric. So molecular will be ionic in nature and we know that water is polar because O will attract the electron cloud towards it (more electronegative) hence there will be formation of dipole (two oppositely charged ions separated by a short distance). Therefore there will be ion-dipole interaction between them.
(iv) methanol and acetone are both polar molecules because of the presence of electron withdrawing O atom in methanol and ketone group in acetone. So they will have dipole-dipole interaction.
(v) acetonitrile is polar compound due to presence of electronegative N atom and acetone due to ketone group. So, dipole-dipole interaction.
Q 2.24 Based on solute-solvent interactions, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN.
Now n-octane is non-polar solvent due to long chain saturated structure. We know that “like dissolves like” so a non-polar compound will be more soluble in non-polar solvent as compared to polar compound.
So cyclohexane is non-polar due to symmetric structure. KCl is ionic in nature as it will dissociate into K + and Cl- ions. CH3CN is polar as mentioned above and CH3OH is also polar in nature.
The order of increasing polarity is:
Cyclohexane < CH3CN < CH3OH < KCl (O is more electronegative than N)
Therefore, the order of increasing solubility is:
KCl < CH3OH < CH3CN < Cyclohexane
Q 2.47 H2S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant
According to Henry's law,"At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
Stated as,
p = KHx
Where, P = partial pressure of the solute above the solution
KH = Henry's constant
x = concentration of the solute in the solution
Given,
Solubility of H2S in water at STP is 0.195 m
We know,
At STP pressure p = 0.987 bar
0.195 mol of H2S is dissolved in 1000g of water
Moles of water = 1000/18
= 55.56 g/mol
∴ the mole fraction of H2S = Moles of H2S / Moles of H2S + Moles of water
= 0.195 / 0.195+55.6
= 0.0035
According to Henry's law, p = KHx
KH = p/x
KH = 0.987 / 0.0035
KH = 282 bar
∴ The Henry’s law constant is 282 bar
Q 2.48 Henry’s law constant for CO2 in water is 1.67x10^8 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
According to Henry's law, "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
Stated as,
p = KHx
Where,
P = partial pressure of the solute above the solution
KH = Henry's constant
x = concentration of the solute in the solution
Given,
KH = 1.67x108 Pa
PCO2 2.5 atm = 2.5 * 1.01325 * 105Pa
According to Henry's law,
p = KHx
x = P/KH
x = 2.5 X 1.01325 X 105 / 1.67 X 108
x = 0.00152
In 500 ml of soda water there is 500 ml of water (neglecting soda)
Mole of water = 500/18
= 27.78 mol
Now,
x = nco2 / nco2 + nH2o
nco2 = x nH2o
= 0.00152 X 27.78
= 0.042 mol
Hence, quantity of CO2 in 500mL of soda water 0.042 * 44 = 1.848 g
Q 2.45 Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
2.5 kg of 0.25 molal aqueous solution.
Molar mass of urea (NH2CONH2) = (2 (1 * 14 + 2 * 1) + 1 * 12 + 1 * 16)
= 60 g/mol
1000 g of water contains 0.25 mol = (0.25 * 60) g of urea.
= 15 g of urea.
Means, 1015 g of solution contains 15 g of urea
Therefore,
2500 g of solution contains = 15 X 2500 / 1015
= 36.95 g
Hence, mass of urea required is 37 g (approx).
Q 2.49 The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K . Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
Given, PAo = 450 mm Hg
PBo = 700 mm Hg
ptotal = 600 mm of Hg
By using Rault's law,
ptotal = PA + PB
ptotal = PAoxA + PBoxB
ptotal = PAoxA + PBo ( 1 - xA )
ptotal = (PAo- PBo)xA + PBo
600 = (450 - 700) xA + 700
-100 = -250 xA
xA = 0.4
∴ xB = 1 - xA
xB = 1 – 0.4
xB = 0.6
Now,
PA = PAoxA
PA = 450 × 0.4
PA = 180 mm of Hg and
PB = PBox
PB = 700 × 0.6
PB = 420 mm of Hg
Composition in vapour phase is calculated by
Mole fraction of liquid,
A =PA / PA + PB
= 180/180+420
= 0.30
Mole fraction of liquid,
B =PB / PA + PB
= 420 / 180+420
= 0.70
Q 2.20 A 5% solution (by mass) of cane sugar in water has freezing point of 271K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
5% solution means 5g of cane sugar is present in 100g of solution
Freezing point of solution = 271k
Freezing point of pure water = 273.15k
Molar mass of cane sugar (C12H22O11) = 12 × 12 + 1 × 22 + 16 × 11 = 342g
Moles of cane sugar = mass/molar mass = 5/342
⇒ n = 0.0146mol
Molality of solution = moles of solute/mass of solvent (in kg)
⇒ M = 0.0146/0.095
⇒ Molality = 0.154M
Depression in freezing point = ΔTf = 273.15-271 = 2.15k
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ Kf = 2.15/0.154
⇒ Kf = 13.96k kg mol-1
Second condition: mass of glucose = 5g
Molar mass of glucose (C6H12O6) = 12 × 6 + 1 × 12 + 16 × 6 = 180g
Moles of glucose = mass/molar mass
⇒ n = 5/180moles
⇒ n = 0.0278mol
Molality of solution = moles of solute/mass of solvent (in kg)
⇒ molality = 0.0278/0.095
⇒ M = 0.2926M
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ ΔTf = 13.96 × 0.2926
⇒ ΔTf = 4.08k
Freezing point of solution = 273.15 + 4.08 = 277.234k
Q 2.33 19.5 g of CH2FCOOH is dissolved in 500 g of water. The depression in the freezing point of water observed is 1.00 C. Calculate the van’t Hoff factor and dissociation constant of fluoroacetic acid.
Given- w1 = 500g
W2 = 19.5g
Kf = 1.86 K kg mol-1
Molar mass of CH2FCOOH = 12 + 2 + 19 + 12 + 16 + 16 + 1
= 78 g mol-1
The depression in freezing point is calculated by,

→ (where, m is the molality)
= 1.86 X 19.5 / 78 X 1000/500
= 1.86 X 19.5 / 78 X 2
=0.93
∴ Δtf (calculated) = 0.93
To find out the vant Hoff’s factor, we use the formula,
i = observed Δtf / calculated Δtf
i = 1.0 (given) / 0.93
∴ i= 1.07
CH2FCOOH → CH2FCOO- + H +
To find out the degree of dissociation α, we use

Thus, the vant Hoff’s factor is 1.07 an the dissociation constant is 2.634x10-3
Q 2.34 Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapour pressure of water at 293 K when 25 g of glucose is dissolved in 450 g of water.
Given- Vapour pressure of water,
PA0 = 17.535 mm Hg
WB= 25 g of glucose
WA = 450g of water
Molar mass of water, H2O = 1 + 1 + 16 = 18 g mol-1
Molar mass of glucose, C6H12O6 = (12*6) + (1*12) + (16*6) = 180 g mol-1
Using Raoult's law for solution of non-volatile solute,
PA0 - PA / PA0 = xB? Equation 1
where xB is the mole fraction of the solute
xB = WB/MB X MB/WB
=25/180 X 18 / 450
xB = 1/180
Substituting the value of xB in equation 1, we get,

Thus, the vapour pressure of water at 293 K at the given conditions is 17.437 mm Hg
Q 2.22 At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars at the same temperature, what would be its concentration?
Here,
T = 300 K
π = 1.52 bar
R = 0.083 bar L
Applying the relation, π = CRT
where
π = osmotic pressure of solution
C = concentration of solution
R = universal gas constant
T = temperature
⇒C = π / RT = 1.52 / 0.083 X 300
⇒ C = 0.061mol/L
Concentration of the solution is 0.061mol/L
Q 2.31 The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the pattern,
Acetic acid< trichloroacetic acid< trifluoroacetic acid.
This is because fluorine is more electronegative than chlorine. So, trifluoracetic acid is a stronger acid in comparison to trichloroacetic acid and acetic acid. And also, acetic acid is the weakest of all.
Explanation: Stronger acid produces more number of ions, therefore it has more? Tf (depression in freezing point), hence lower freezing point. As the acidic strength increases, the acid gets more and more ionised.
Trifluoracetic acid ionizes to the largest extent. Hence, in this case, trifluoracetic acid being the strongest acid produces more number of ions (extent of ionisation and concentration of ions are more), high? Tf (depression in freezing point)and lower freezing point and vice versa.
Q 2.43 Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Let the total mass of the solution be 100 g and the mass of benzene be 30 g.
∴ Mass of carbon tetrachloride = (100 - 30) g = 70 g
Molar mass of benzene (C6H6) = (6 × 12 + 6 × 1) g mol -1
= 78 g mol -1
∴ Number of moles of C6H6 =30/78 mol
= 0.3846 mol
Molar mass of carbon tetrachloride (CCl4) = 1 × 12 + 4 × 35.5
= 154 g mol -1
∴ Number of moles of CCl4 = 70/154 mol
= 0.4545 mol
Thus, the mole fraction of C6H6 is given as:
Number of moles of C6H6 / Number of moles of C6H6 + Number of moles of CCl4
= 0.3846 / (0.3846 + 0.4545)
Q 2.28 Calculate the mass percentage of aspirin (C9H8O4 ) in acetonitrile (CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN.
Total mass of solution = 6.5g + 450g = 456.5g
Therefore mass percentage of aspirin in solution = (mass of aspirine/total mass of solution) = 6.5/456.5
⇒ mass % of aspirine = 1.424%
Q 2.40 Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27° C.
Given-
Vant Hoff’s factor, I = 2.47
osmotic pressure, π = 0.75 atm
Volume of solution = 2.5L.
To determine the amount of CaCl2, we use vant Hoff’s equation for dilute solutions, given as,
πV = inRT
where, n is the number of moles of solute, R is solution constant which is equal to the gas constant and T is the absolute temperature.

Hence, the amount of CaCl2 dissolved is 3.425g
Intext Q 2.42 Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Mass of Solution = Mass of Benzene + Mass of Carbon Tetrachloride
= 22 g + 122 g = 144 g
Mass percentage of Benzene = Mass of Benzene / Mass of Solution X 100 = 22/144 X 100 = 15.28%
Mass percentage of CCl4 = Mass of CCl4 / Mass of Solution X 100 = 122/144 X 100 = 84.72%
Q 2.37 Vapour pressures of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot ptotal, pchloroform, and pacetone as a function of xacetone. The experimental data observed for different compositions of mixture is: Plot this data also on the same graph paper. Indicate whether it has positive deviation or negative deviation from the ideal solution.
|
100 xacetone |
0 |
3.4.8 |
23.4 |
36.0 |
50.8 |
58.2 |
64.5 |
72.1 |
|
pacetone /mm Hg |
0 |
54.9 |
110.1 |
202.4 |
322.7 |
405.9 |
454.1 |
521.1 |
|
pchloroform /mm Hg |
632.8 |
548.1 |
469.4 |
359.7 |
257.7 |
193.6 |
161.2 |
120.7 |
The Ptotal for the values given in the graph is found out and plotted in the graph.
ptotal (mm Hg) | 632.8 | 603.0 | 579.5 | 562.1 | 580.4 | 599.5 | 615.3 | 641.8 |

It can be observed from the graph that the plot for the p total of the solution curves downwards. Therefore, the solution shows negative deviation from the ideal behaviour.
Q 2.36 100 g of liquid A (molar mass 140 g mol^–1) was dissolved in 1000 g of liquid B (molar mass 180 g mol^–1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 Torr.
Given-
Mass of liquid A, WA = 100g, Molar mass, MA = 140 g mol-1
Mass of liquid B, WB = 1000 g, Molar mass, MB = 180 g mol-1
Using the formula below calculate the no. of moles in liquid A and B.
Number of moles = Mass / Molar Mass
Number of moles of liquid A, MA = 100/140 = 0.714 mol-1
Number of moles of liquid B, MB = 1000/ 180 = 5.556 mol-1
Using the formula,
mole fraction of a liquid = No. of moles of the liquid / total no of moles
we calculate the mole fraction of liquids A and B.
→ Mole fraction of A,
xA = 0.714 / (0.714 + 5.556)
∴ xA = 0.114
→ Mole fraction of B,
xB = 1- xA = 1 - 0.114
∴ xB = 0.886
Vapour pressure of pure liquid B, Po = 500 torr (given)
According to Henry's law,

Having given the total vapour pressure of the solution, Ptotal = 475 torr,

Thus, the vapour pressure of pure liquid A = 280.7 torr and
vapour pressure of liquid A in the solution = 32 torr
Q 2.46 Calculate (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is 1.202 g mL^-1 .
(a) Molality, also called molal concentration, is a measure of the concentration of a solute in a solution in terms of amount of substance in a specified amount of mass of the solvent. Molar mass of KI = 39 + 127 = 166 g/mol.
20% aqueous solution of KI means 200 g of KI is present in 1000 g of solution. Therefore,
Molality = Moles of KI / Mass of Water in kg
= (200/166) / (0.8) = 1.506 m
(b) Molarity is the concentration of a solution expressed as the number of moles of
solute per litre of solution.
Given,
Density of the solution = 1.202 g/mL
Volume of 100 g solution = mass/ density
= 100/1.202
= 83.19 mL
Therefore, molarity = 20/166 mol/ 83.19 × 10-3 L
= 1.45 M
∴ Molarity of KI = 1.45M
(c) Molar mass of KI = 39 + 127 = 166 g/mol.
Moles of KI = 20/166 = 0.12 mol
Moles of water = 80/18 = 4.44 mol
Therefore,
Mole fraction of KI = Moles of KI / Moles of KI+ Moles of Water
= 0.12 / 0.12+ 4.44
= 0.0263
∴ Mole fraction of KI = 0.0263
Q 2.18 Calculate the mass of a non-volatile solute (molar mass 40 g mol^–1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
Given:
Molar mass of non-volatile solute = 40g
Let no. of moles of solute be n.
Mass of octane = 114g
Molar mass of octane (C8H18) = 12 × 8 + 1 × 18 = 114g/mol
Moles of octane = given mass/molar mass
⇒ n = 114/114 moles
⇒ n = 1 mole
Molar fraction of solute,
x2 = moles of solute / moles of solute + moles of octane
⇒ x2 = n/n + 1
Let the vapour pressure of original solvent (without solute) be p1?
Accordingly after addition of solute vapour pressure of solution reduces to 80% i.e.
0.8 p1? = p1
Applying the formula:

⇒ n/n + 1 = 0.2
⇒ 0.2n + 0.2 = n
⇒ n = 0.25 moles
Hence, mass of solute is:
moles = given mass/molar mass
⇒ 0.25moles = mass/40g
⇒ mass = 10g.
Q 2.39 The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if the Henry’s law constants for oxygen and nitrogen at 298 K are 3.30 × 10^7 mm and 6.51 × 10^7 mm respectively, calculate the composition of these gases in water.
Given-
KH for O2 = 3.30 * 107 mm Hg,
KH for N2 = 6.51 * 107 mm Hg
Percentage of oxygen (O2) = 20 %
Percentage of nitrogen (N2) = 79%
Total pressure = 10 atm
Using Henry's law,

where, p is the partial pressure of gas in the solution and KH is Henry's constant.

Thus, the mole fraction of oxygen in solution, xoxy = 4.61x10-5
and the mole fraction of nitrogen in solution, xnit is 9.22x10-5
Q 2.32 Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. Ka = 1.4 × 10^–3 , Kf = 1.86 K kg mol^–1 .
Mass of CH3CH2CHClCOOH = 10 g
Mass of water = 250g
Ka = 1.4 × 10–3,
Kf = 1.86 K kg mol–1
Molar mass of CH3CH2CHClCOOH = 12 + 3 + 12 + 2 + 12 + 1 + 35.5 + 2 + 16 + 16 + 1
= 122.5 g mol–1
Number of moles of solute = Mass of Solute / Molar Mass
→ No. of moles = 10g / 122.5 g/mol
∴ No. of moles = 8.6 X 10–2 mol
Now, Molality is given as,
M = Number of moles of solute / kg of solvent
M= 8.6 X 10–2 X 1000 g/mol / 250 g
M = 0.3264 kg/mol
CH3CH2CHClCOOH = CH3CH2CHClCOO- + H +
Initial moles | 1 | 0 | 0 |
Equilibrium moles |
(1-α) |
α |
α
|
Total moles at equilibrium = (1-α) + 2 α
= 1 + α
In order to find out the depression in freezing point,

values of I (vant Hoff’s factor) and α (degree of dissociation) are to be found out.
To find out degree of dissociation, α

∴ I = 1.0654
Now, to find out the depression in freezing point,

Q 2.38 Benzene and toluene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at 300 K are 50.71 mm Hg and 32.06 mm Hg respectively. Calculate the mole fraction of benzene in vapour phase if 80 g of benzene is mixed with 100 g of toluene.
Given-


Thus, mole fraction of benzene in vapour phase is 0.6744
Q 2.51 Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.
Given,
Mass of water, wl = 500 g
Boiling point of water = 99.63°C (at 750 mm Hg).
Molal elevation constant, Kb = 0.52 K kg/mol
Molar mass of sucrose (C12H22O11), M2 (11 × 12 + 22 × 1 + 11 × 16) = 342 g/mol
Elevation of boiling point ΔTb = (100 + 273) - (99.63 + 273) = 0.37 K
We know that,
ΔTb = Kb X 1000 X W2 / M2 X W1
0.37 = 0.52 X 1000 X W2 / 340 X 500
w2 = 0.37 X 342 X 500 / 0.52 X 1000
w2 = 121.67 g
Hence,
121.67 g (approx) Sucrose is added to 500g of water so that it boils at 100°C.
Q 2.21 Two elements A and B form compounds having formula AB2 and AB4 . When dissolved in 20 g of benzene (C6H6 ), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol^–1. Calculate atomic masses of A and B
let the molar masses of AB2 and AB4 be x and y respectively.
Molar mass of benzene (C6H6) = 12 × 6 + 1 × 6 = 78 g/mol
Moles of benzene = mass/molar mass = 20/78
n = 0.256mol
⇒ ΔTf = 2.3 K
Kf = 5.1K kg mol-1
For AB2
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 2.3 = 5.1 × M1
⇒ M1 = 0.451mol/kg
For AB4
Applying the formula: ΔTf = Kf × M
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 1.3 = 5.1 × M2
⇒ M2 = 0.255mol/kg
M1 = moles of solute/mass of solvent (in kg)
M1 = 1/x / 0.02 = 1 / 0.02x = 0.451
⇒ X = 110.86g
M2 = moles of solute/mass of solvent (in kg)
M2 = 1/y / 0.02 = 1 / 0.02y = 0.255
⇒ y = 196.1g
atomic mass of A be a and that of B be b g respectively.
So, AB2 : a + 2b = 110.86
AB4: a + 4b = 196.1
⇒ a = 25.59g
⇒ b = 42.64g
Q 2.27 If the solubility product of CuS is 6 × 10^–16, calculate the maximum molarity of CuS in aqueous solution?
The Solubility product of CuS (ksp) = 6 × 10-16
CuS → Cu ++ + S2-
Let the s be solubility of CuS in mol/L
Ksp = [ Cu ++ ] [S2]
Ksp = solubility product
6 × 10-16 = s × s = s2
⇒ S = 2.45 × 10-8 mol/L
Hence, the maximum molarity of CuS in an aqueous solution is 2.45 × 10-8 mol/L
Q 2.35 Henry’s law constant for the molality of methane in benzene at 298 K is 4.27 × 10^5 mm Hg. Calculate the solubility of methane in benzene at 298 K under 760 mm Hg.
Given-
Henry's law constant KH = 4.27X 105 mm Hg,
p = 760mm Hg,
Using Henry's law,

Using the formula of lowering vapour pressure,

Thus, the solubility of methane in benzene is 0.023 moles
Q 2.30 Calculate the amount of benzoic acid (C6H5COOH) required for preparing 250 mL of 0.15 M solution in methanol.
Volume of the solution = 250mL = 0.25L
Let the no. of moles of solute be n
Molarity = No. of moles of solute/volume of solution
⇒ 0.15 = n/0.25
⇒ n = 0.0375moles
Molar mass of C6H5OH = 6×12 + 5×1 + 16 + 1 = 94g
Moles = mass/molar mass
⇒ 0.0375 = m/94
Mass of benzoic acid required = 3.525g.
Q 2.25 Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water? (i) phenol (ii) toluene (iii) formic acid (iv) ethylene glycol (v) chloroform (vi) pentanol.
- Water is a polar compound (due to electronegativity difference between O and H) . We know that “like dissolves like”. So, a non-polar compound will be more soluble in non-polar solvent as compared to polar compound.
- Phenol has the polar group -OH and non-polar group –C6H5 and it can not form H bonding with water (presence of bulky non-polar group) . Thus, phenol is partially soluble in water
- Toluene has no polar Thus, toluene is insoluble in water.
- Formic acid (HCOOH) has the polar group -OH and can form H-bond with water. Thus, formic acid is highly soluble in water
- Ethylene glycol (OH-CH2-CH2-OH) has polar -OH group and can form H-bond with Thus, it is highly soluble in water.
- Chloroform is partly soluble as CHCl3 is polar in nature due to high electronegativity of Cl atoms, there will be production of partial + charge on H atom so it can form H bonding with water but it is also surrounded by 3 Cl atoms, so partly soluble
- Pentanol (C5H11OH) has polar -OH group, but it also contains a very bulky non- polar group –C5H11. Thus, pentanol is partially soluble in water
Q 2.29 Nalorphene (C19H21NO3 ), similar to morphine, is used to combat withdrawal symptoms in narcotic users. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 × 10^–3 m aqueous solution required for the above dose.
Molar mass of Nalorphene = 311g/mol
Now 1000g of solution contains 1.5 × 10-3 moles of Nalorphene (Molality of solution = moles of solute/mass of solvent (in kg)
⇒ 1.5 × 10-3 moles of Nalorphene = 1.5 × 10-3 × 311 = 0.4665g of Nalorphene
Therefore, total mass of the solution = (1000 + 0.4665) g
⇒ total mass = 1000.4665 g
This implies that the mass of the solution containing 0.4665 g of nalorphene is 1000.4665 g.
Therefore, mass of the solution containing 1.5 mg of nalorphene is:
Mass = 1000.4665 X 1.5 X 10-3 / 4.665 g
⇒ mass of solution containing required ions = 3.22 g
Hence, the mass of aqueous solution required is 3.22 g.
Complete Class 12 Chemistry Solutions Study Material
Your one-stop solution for all the requirements to clear the Class 12 Chemistry exam with excellent marks is here. The given table consists of all that you need, including assignment, study notes, NCERT Solutions, NCERT Notes, quick revision notes and important formulas for Chapter 1 Solutions.
| Particular | Link |
|---|---|
| Class 12 Chemistry Solutions Notes | Download Here |
| Chapter 1 Solutions Quick Revision Notes | Download Here |
| Chemistry Solutions Important Formulas | Download Here |
| Solutions Important Questions for CBSE, JEE, and NEET | Download Here |
| Class 12 Chapter 1 NCERT Exemplar Solutions | Download Here |
Class 12 Chemistry Chapter 1 Solutions Important Topics
This chapter covers fundamentaly important topics to understand liquid solutions and formations, colligative properties, vapour pressure and osmosis. Check the list of key topics below.
Key Topics in Class 12 Chemistry Chapter 1 Solutions
- Solutions and Their Types
- Concentration of Solutions
- Solubility
- Solubility of a Solid in a Liquid
- Solubility of a Gas in a Liquid
- Henry’s Law
- Vapour Pressure of Pressure of Pressure of Liquid Solutions
- Vapour Pressure of Liquid - Liquid Solutions
- Vapour Pressure of Solid - Liquid Solutions
- Raoult’s Law
- Ideal and Non-ideal Solutions
- Colligative Properties
- Relative Lowering of Vapour Pressure
- Elevation of Boiling Point
- Depression of Freezing Point
- Determination of Molar Mass
- Osmosis and Osmotic Pressure
- Reverse Osmosis and Water Purification
Chemistry Class 12 Chapter 1 Solutions Important Formula Sheet
Important Formulae of Solutions for CBSE and Competitive Exams
- Concentration Terms
| Important Concentration Terms Formulae | |
|---|---|
| Mass Percentage (%) | |
| Mole Fraction ( ) | |
| Molarity (M) | |
| Molality (m) | |
| Normality (N) | |
- Colligative Properties
- Relative Lowering of Vapour Pressure
-
- Elevation of Boiling Point ( )
= Molal elevation constant
-
-
Depression of Freezing Point
-
= Molal depression constant
-
-
Osmotic Pressure ( )
-
-
- Where,
= Molarity, = Gas constant (0.0821 L atm/mol K), = Temperature
- Abnormal Molar Mass & Van’t Hoff Factor (
)
- If the observed molar mass differs from the expected value.
- Corrected Colligative Property Formulae
-
Boiling Point Elevation:
-
Freezing Point Depression:
-
Osmotic Pressure:
-
-
- Solubility and Henry’s Law
-
-
Raoult’s Law (Vapour Pressure of Solutions)
-
(Total vapour pressure:
Class 12 Chemistry Solutions: Weightage in CBSE , JEE and NEET
The Class 12 Chemistry Chapter 1 Solutions is important for understanding concentration terms, colligative properties, Raoult’s Law, and ideal/non-ideal solutions. The Chemistry Chapter 1 Class 12 is crucial for Competitive Exams. Check the topics covered in this chapter:
Chemistry Chapter 1 Solutions Weightage in NEET, JEE Main Exam
In both these competitive exams, this chapter holds moderate weightage. Refer to the table below to see the weightage:
| Exam | Number of Questions |
|---|---|
| CBSE | 4 - 7 Marks |
| NEET | 1-2 questions |
| JEE Main | 1-2 questions |
Important Questions of Solutions Class 12 Chemistry
Chapter 1 Solutions Important Questions
What is the mole fraction of a component in a solution?
Various quantitative tools are used to find the concentration of solutions. One of the methods is "Mole Fraction", as its name suggests, it is basically a ratio for the number of moles of a component to the total number of moles.
It is defined as the ratio of the number of moles of the component to the total number of moles of all components present in the solution.
Suppose there are 3 components and the mole fraction for each of the components is 1/3. Now, if we add the mole fraction of all the components, the answer will be 3/3, that is 1. That's why the sum of the mole fraction of all components in a solution is always 1.
What is Henry’s law for the solubility of gases in liquids?
The solubility of gases increases with an increase in pressure. Henry's law provides a good mathematical explanation for variation in solubility in liquid solutions. As per the NCERT textbooks,"
At a constant temperature, the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid.
Mathematically, it is expressed as:
Where:
- x = Mole fraction of the dissolved gas in the liquid
- P = partial pressure of the gas above the liquid
- Kh = Henry’s law constant
What is the van’t Hoff factor (𝑖)? How does it affect the colligative property calculations for electrolyte solutions?
In general, the observational values of colligative properties are not the same as the ideally calculated values. The van't hoff factor helps in understanding the variation of the actual experimental value from the calculated value.
The van’t Hoff factor is the factor that tells the extent of dissociation or association in a solution. It is defined as the ratio of normal molar mass to the abnormal molar mass of the component/solution.
i = Observed colligative property/ Calculated colligative property
Electrolytes (like NaCl or other salts) dissociate into multiple ions in the solutions. This increases the number of solute particles and it results in van't hoff factor not equal to 1, and you can observe the colligative effects.
A solution is prepared by dissolving 5.85 g of NaCl in 500 g of water. Calculate the molality of the solution.
You can check the solutions below.
Class 12 Chemistry Study Material
Commonly asked questions
Why you should use Shiksha's NCERT Solutions for Chapter 1 Solutions Class 12 Chemistry?
Shiksha focuses on completely fulfilling the needs of CBSE class 12 board students. We provide NCERT Solutions for classes 11th and 12th in class for physics, chemistry and mathematics.
Here are the key reasons why you should use Shiksha's NCERT Solutions for the Solution Chapter:
- Complete coverage of NCERT Exercise, including MCQs, very short, short and long answer type questions.
- Step-by-step solutions according to the CBSE board exam needs.
- Intext question and answer with a free downloadable PDF
- complete study material for the chapter
- NCERT Chapter 1 Solutions Notes
- Solutions Quick Revision Notes
- Important Questions for Solution Chapter
- Chemistry Chapter 1 Solutions NCERT Exemplar Solutions
What are the benefits of using Class 12 Chemistry Chapter 1 NCERT Solutions?
NCERT offers the foundation for every class 12 student. CBSE board exam questions are primarily based on the NCERT exercise, Examples and conceptual explanation.
There are various benefits of using NCERT Solutions.
- Specially for CBSE Board students, these NCERT Solutions are the best resource to prepare for the boards.
- These NCERT Solutions include step-wise descriptive answer approach that will help you write good answers.
- These solutions also help you understand problem-solving and how to approach a specific type of conceptual or numerical problem.
- If you are preparing for the JEE or NEEt exam, these solutions can be your first decisive step towards success.
- For NEET and JEE aspirants, NCERT solutions are the first foundation block that offer understanding of fundamental concepts, their application
What is the weightage of Class 12 Chapter 1 Solutions in CBSE and other competitive exams?
After the 2023-24 revised CBSE syllabus, many chapters have been removed, and in some chapters syllabus is reduced. As per the latest CBSE exam pattern 2025-26, the Chapter 1 Solutions will be asked for 5-7 marks in the Class 12 Chemistry CBSE board exams.
For other competitive exams, the Solutions chapter has a medium weightage in the NEET exam. In the NEET exam, 1-2 questions are frequently asked based on the concept of moderate-level numerical.
Physical Chemistry holds over 30% weightage in the chemistry section in the JEE Mains exam. The solutions chapter is considered a part of physical chemistry. You can expect 1 question form the solutions chapter in JEE Mains exam.
Explore exams which ask questions on Chemistry Ncert Solutions Class 12th
Select your preferred stream
Chemistry Ncert Solutions Class 12th Exam