Organic compounds are, at their core, carbon-based molecules in which carbon atoms form the backbone of the structure, usually by bonding to hydrogen, oxygen, nitrogen, halogens, or other carbons. They differ from “inorganic” substances (like salts or minerals) by this central role of carbon–carbon (C–C) and carbon–hydrogen (C–H) bonding. The topic forms the foundation of organic chemistry which makes it an important topic. Students who want to pass class 12th Science stream with flying colours must practice NCERT solutions of the chapter.
- Classification of Organic Compounds
- Classification of Organic Compounds based on the kind of atoms
- Classification of Organic Compounds Based on Functional Groups
Classification of Organic Compounds
Organic compounds are classified into two types based on the skeleton of the carbon chain:
- Acyclic or Open-chain compounds
- Cyclic or Closed-chain compounds
Acyclic or Open-chain compounds:
The compounds in which the carbon atoms form a chain-linked in a linear pattern or branched are called as acyclic or open-chain compounds.
Open-chain compounds are further classified as:
(i) Saturated compounds: The compound is saturated if single bonds connect all of the carbon atoms in the chain. In this, all the valence of carbon are satisfied by single bonds.
(ii) Unsaturated compounds: If there are double or triple bonds between the carbon atoms, the compound is unsaturated. In this, all the valence of carbon are satisfied by multiple bonds.
Cyclic or Alicyclic or Closed-chain or Ring compounds:
1.It has two types including:
(i) Heterocyclic compounds: the chain contains carbon as well as other atoms such as oxygen, nitrogen, sulphur, etc.
(ii) Homocyclic compounds (Carbocyclic compounds): Those type of organic compounds in which the chain contains only carbon atoms.
Carbocyclic compounds are further classified as:
(i) Alicyclic compounds: Which have one or more carbocyclic rings that can be either saturated or unsaturated.
(ii) Aromatic compounds: Chemical compounds that consist of conjugated planar ring systems accompanied by delocalized pi-electron clouds in place of individual alternating double and single bonds. It contains hybridized carbon atoms and must obey the Huckel rule.
a) Benzenoid aromatic compounds
Benzenoid compounds are molecules that have at least one benzene ring in its chemical structure. A benzene ring is a cyclic structure having six carbon atoms as the ring members. It has three pi bonds (double bonds) and three sigma bonds arranged in an alternative pattern. Therefore, we call this pattern a conjugated pi (π) system. Since the molecule has double bonds due to benzene ring, the molecule is an unsaturated compound with extra stability provided by the conjugated pi (π) system. (Examples: Aniline, Naphthalene, anthracene, diphenyl methane
b) Non-benzenoid aromatic compounds
Non benzenoid compounds are aromatic compounds that having conjugated systems with planar cyclic structure do not have benzene rings in their structure. Examples: Azulene and Thiophene.
Do note that this topic is important for competitive exams such as NEET and JEE Main amongst others in India.
Classification of Organic Compounds based on the kind of atoms
Hydrocarbons:
Only hydrogen and carbon atoms make up the organic molecules known as hydrocarbons. The structure of the compounds is produced by the connecting of carbon atoms. According to the hypothesis, by trading one or more hydrogen atoms for other atoms or groups of atoms, all other compounds may be made from these. These are referred to as the parents of the organic compounds.
Characteristics of Hydrocarbons:
- Lower hydrocarbons, such as methane and ethane, are gases at room temperature.
- Hydrocarbons have no colour and no odour.
- The boiling point of hydrocarbons shoots up as the number of carbon atoms increases.
- Hydrocarbons undergo a combustion reaction with oxygen, resulting in the formation of CO 2 and water.
- When compared to other classes of hydrocarbons, alkanes are the least reactive. Because of the presence of the triple bond, alkynes are the most reactive.
- Alkanes are saturated compounds, whereas alkenes and alkynes are unsaturated compounds.
- Hydrocarbons are not soluble in water.
Hydrocarbons are further classified into three classes:
i. Alkanes
ii. Alkenes
iii. Alkynes
1. ALKANE
- Alkanes are saturated hydrocarbons; they only contain single bonds. They are called “saturated” because they contain the maximum amount of hydrogen atoms possible.
- The general formula for an alkane is where is a positive integer.
In other words:
- Alkanes are a series of compounds that contain carbon and hydrogen atoms with single covalent bonds.
- Alkanes have the general chemical formula . (sometimes called the parent molecule).
- In an alkane, each carbon atom is -hybridized with 4 sigma bonds (either C – C or C – H), and each hydrogen atom is joined to one of the carbon atoms (in a C – H bond) and has a tetrahedral shape, with the bonded atoms at angles of to each other.
- The longest series of linked carbon atoms in a molecule is known as its carbon skeleton or carbon backbone.
- The number of carbon atoms may be considered as the size of the alkane.
- The list of some Alkanes with the molecular formula is as follows:
| Name | Molecular Formula |
|---|---|
| methane | |
| ethane | |
| propane | |
| butane | |
| pentane | |
| hexane | |
| heptane | |
| octane | |
| nonane | |
| decane |
2. Alkene
Alkenes are organic compounds that contain one double bond between carbon-carbon atoms. They are a series of unsaturated compounds with a general molecular formula .
| No. of C atoms | Name of alkene | Molecular formula |
|---|---|---|
| 2 | Ethene | |
| 3 | Propene | |
| 4 | Butene | |
| 5 | Pentene | |
| 6 | Hexene | |
| 7 | Heptene | |
| 8 | Octene | |
| 9 | Nonene | |
| 10 | Decene |
iii. ALKYENE
Alkynes are hydrocarbons with carbon-to-carbon triple bonds . Alkynes are unsaturated due to the presence of a triple bond between carbon atoms.
- Alkynes are represented by the general formula .
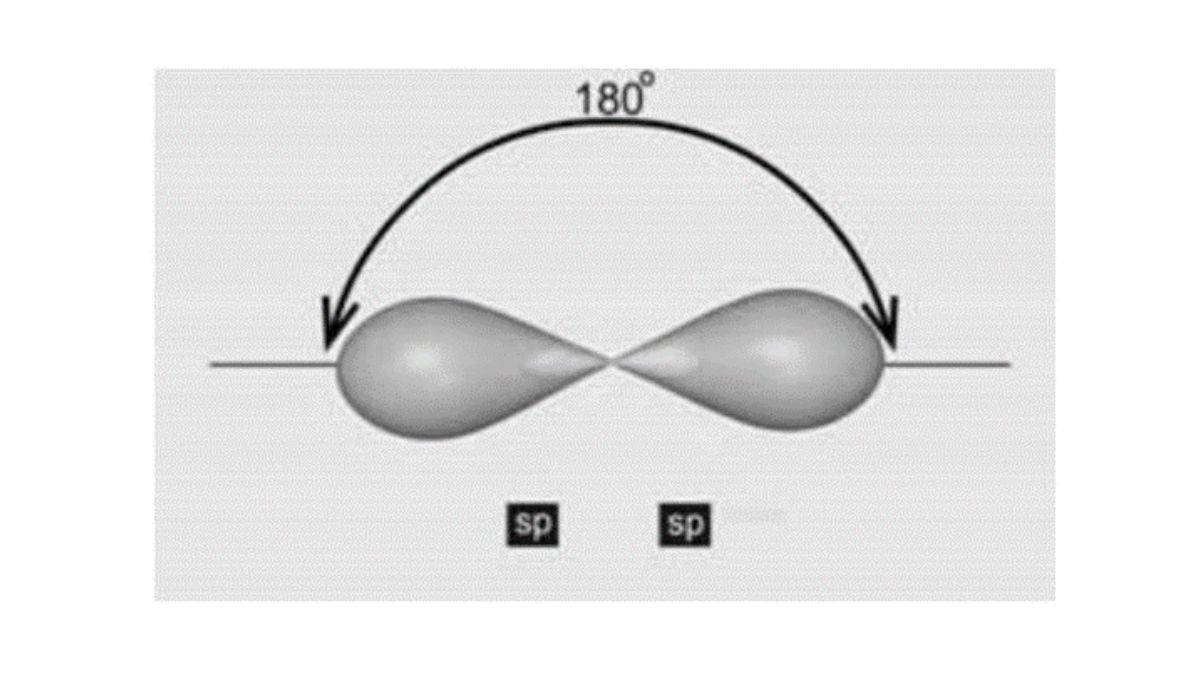
- Alkynes undergo hybridization and the hybrid orbitals are oriented in opposite directions, forming an angle of with each other.
| Name | Open structure |
|---|---|
| Ethyne | |
| Propyne | |
| Butyne | |
| Pentyne | |
| Hexyne |
Hydrocarbons with 1 to 5 carbon atoms:
| Number of carbon atoms |
Alkane (CnH2n+2) |
Alkene (CnH2n) |
Alkyne (CnH2n-2) |
| 1 |
Methane ( CH4 ) |
- |
- |
| 2 |
Ethane ( C2H6 ) |
Ethene ( C2H4 ) |
Ethyne ( C2H2 ) |
| 3 |
Propane ( C3H8 ) |
Propene ( C3H6 ) |
Propyne ( C3H4 ) |
| 4 |
Butane ( C4H10 ) |
Butene ( C4H8 ) |
Butyne ( C4H6 ) |
| 5 |
Pentane ( C5H12 ) |
Pentene ( C5H10 ) |
Pentyne ( C5H8 ) |
Note: Methene and Methyne do not exist because they have only one carbon atom. These compounds cannot form multiple bonds, as hydrogen can share only one electron.
Atoms like hydrogen, oxygen, nitrogen, and others that form bonds with carbon are present in organic molecules in addition to carbon. Different kinds of organic molecules are created when these atoms join with carbon.
Note: Methene and Methyne do not exist because they have only one carbon atom. These compounds cannot form multiple bonds, as hydrogen can share only one electron.
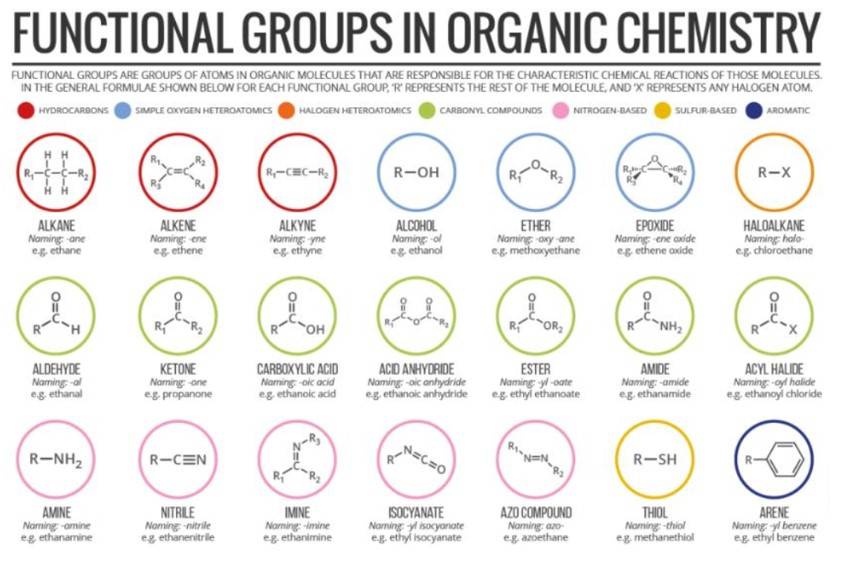
Classification of Organic Compounds Based on Functional Groups
1. The structural frameworks of organic compounds are composed of carbon and hydrogen, which are less reactive. However, the presence of another atom or group of atoms makes the compounds more reactive, determining the chemical properties of the compounds. These are known as functional groups.
2. The chemical properties of organic compounds are determined by its functional group, whereas the remaining structure of the compound determines its physical properties.
3. Various classes or families of organic compounds are shown in the table below, along with their functional groups:
Chemistry Organic Chemistry Exam
Student Forum
Other Topics under this Chapter
- Overview
- Classification of Organic Compounds
- Tetravalence of Carbon Shapes of Organic Compounds
- Structural Representations of Organic Compounds
- Nomenclature of Organic Compounds
- Isomerism
- Fundamental Concepts in Organic Reaction Mechanism
- Methods of Purification of Organic Compounds
- Qualitative Analysis of Organic Compounds
- Quantitative Analysis
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics