Organic Chemistry Class 11 NCERT Solutions talks about the organic compounds that are vital for sustaining life on Earth. It includes DNA and proteins, which are essential parts of the muscles, blood, and skin. Organic compounds appear in materials like fuels, clothing, dyes, polymers, and medicines.
After studying the Organic Chemistry Class 11, the students will be able to write the structures of the organic molecules, understand the reasons for the tetravalence of carbon and shapes of organic molecules, classify the organic compounds, understand the organic reaction mechanism, and more related concepts.
To get the NCERT notes of all the chapters of Class 11 Chemistry, its important topics, PDFs, and solved examples, the students should check here.
- Class 11 Organic Chemistry: Topics Covered, Chapter Weightage
- Topics Covered in Organic Chemistry- Some Basic Principles and Techniques Chapter
- NCERT Solutions for Class 11 Chemistry Chapter 8 – Free PDF on Organic Chemistry
- Organic Chemistry - Some Basic Principles and Techniques Structure Solutions
- Organic Chemistry - Some Basic Principles and Techniques Structure More Solutions
- Organic Chemistry - Some Basic Principles and Techniques Structure Additional Questions
Class 11 Organic Chemistry: Topics Covered, Chapter Weightage
Organic Chemistry Class 11 NCERT Solutions is an important chapter for competitive exams. The topics on which students should focus include: Hydrocarbons, General Organic Chemistry, Aldehydes, Ketones, and Carboxylic Acids, Alcohols, Phenols, and Ethers, and Biomolecules. See here the topics covered in the organic chemistry class 11:
| Exercise | Topics Covered |
|---|---|
| 8.1 | General Introduction |
| 8.2 | Tetravalence of Carbon: Shapes of Organic Compounds |
| 8.3 | Structural Representations of Organic Compounds |
| 8.4 | Classification of organic compounds |
| 8.5 | Nomenclature of organic compounds |
| 8.6 | Isomerism |
| 8.7 | Fundamental Concepts in Organic Reaction Mechanism |
| 8.8 | Methods of Purification of Organic Compounds |
| 8.9 | Qualitative Analysis of Organic Compounds |
| 8.10 | Quantitative Analysis |
Organic Chemistry Some Basic Principles and Techniques Weightage in NEET and JEE Mains Exams
| Exam | Number of Questions | Weightage |
|---|---|---|
| NEET | 15-16 questions | 33-36% |
| JEE Main | 3-4 questions | 33.33% |
Important Reaction of Organic Chemistry- Some Basic Concepts and Techniques for Competitive and CBSE Exams
- Substitution Reactions (SN1 & SN2): A reaction where one functional group replaces another in a molecule.
- Addition Reactions: Addition of atoms/groups to unsaturated compounds (alkenes, alkynes).
(Markovnikov’s Rule - H⁺ adds to the carbon with more hydrogens
- Elimination Reactions: Removal of atoms/groups leading to the formation of double/triple bonds.
- Homolytic and Heterolytic Cleavage Reactions
-
- Free Radical Substitution (Halogenation of Alkanes)
- Wurtz Reaction (Alkane Formation)
- Friedel-Crafts Alkylation
(In presence of AlCl₃ catalyst)
Important Formulae of Organic Chemistry-Some Basic Concepts and Techniques
-
Degree of Unsaturation:
(C = Carbon, H = Hydrogen, N = Nitrogen, X = Halogens)
-
Empirical & Molecular Formula:
Topics Covered in Organic Chemistry- Some Basic Principles and Techniques Chapter
Students can check below the list of all topics that are covered in the Chemistry Class 11th NCERT Solutions Chapter 8 - Organic Chemistry: Some Basic Principles and Techniques.
- General Introduction
- Tetravalence of Carbon: Shapes of Organic Compounds
- Structural Representations of Organic Compounds
- Complete, Condensed and Bond-line Structural Formulas
- Three-dimensional Representation of Organic Molecules
- Classification of Organic Compounds
- Nomenclature of Organic Compounds
- The IUPAC System of Nomenclature
- Iupac Nomenclature of Alkanes
- Nomenclature of Organic Compounds Having Functional Group(S)
- Nomenclature of Substituted Benzene Compounds
- Isomerism
- Structural Isomerism
- Stereoisomerism
- Fundamental Concepts in Organic Reaction Mechanism
- Fission of a Covalent Bond
- Nucleophiles and Electrophiles
- Electron Movement in Organic Reactions
- Electron Displacement Effects in Covalent Bonds
- Inductive Effect
- Resonance Structure
- Resonance Effect
- Electromeric Effect (E Effect)
- Hyperconjugation
- Types of Organic Reactions and Mechanisms
- Methods of Purification of Organic Compounds
- Sublimation
- Crystallisation
- Distillation
- Differential Extraction
- Chromatography
- Qualitative Analysis of Organic Compounds
- Detection of Carbon and Hydrogen
- Detection of Other Elements
- Quantitative Analysis
- Carbon and Hydrogen
- Nitrogen
- Halogens
- Sulphur
- Phosphorus
- Oxygen
NCERT Solutions for Class 11 Chemistry Chapter 8 – Free PDF on Organic Chemistry
Students get the link to a free Organic Chemistry Class 11 PDF here. They must download the PDF. The Class 11 students can rely on these solutions for their exam preparation. It is ideal for school exams, CBSE Board and entrance exams like NEET and JEE Mains.
Class 11 Chemistry Chapter 8 Organic Chemistry-Some Basic Concepts and Techniques NCERT Solution PDF: Download Free PDF
Related Links
| NCERT Notes for Class 11 & 12 | Class 11th NCERT Solutions Chemistry | NCERT Solutions Class 11 and 12 for Maths, Physics, Chemistry |
Organic Chemistry - Some Basic Principles and Techniques Structure Solutions
Here we have provided NCERT class 11 chemistry chapter 12 solutions. Having the Organic chemistry basics knowledge is important to solve these questions, otherwise, students might get focused. Students whose Fundamental concepts of organic chemistry are clear will find the problem to be quite easy. Solve the questions and do the self-assessment.
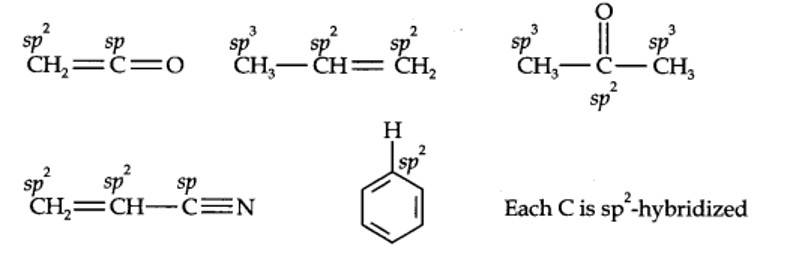
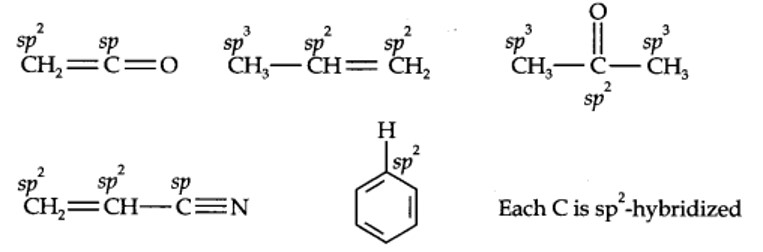
| 12.1. What are the hybridisation states of each carbon atom in the following compounds? CH2=C=O, CH3CH=CH2, (CH3)2CO, CH2=CHCN, C6H6. |
| Answer:
|
| 12.2. Indicate the σ and π-bonds in the following molecules: C6H6, C6H12, CH2Cl2, CH=C=CH2, CH3NO2, HCONHCH3 |
| Answer: Single bond contains only one sigma bond and double bond contains one sigma and one pi bond.
|
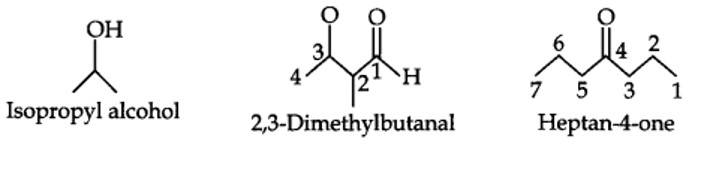
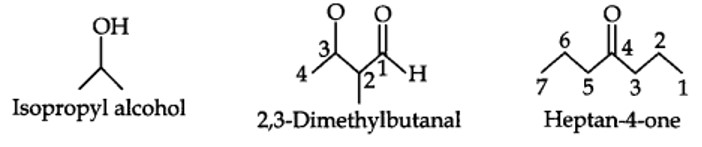
| 12.3. Write bond-line formulas for: Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one. |
| Answer:
|
| 12.4. Give the IUPAC names of the following compounds
|
| Answer: (a) Propylbenzene (b) 3-Methylpentanenitrite (c) 2, 5-Dimethylheptane |
| 12.5.Which of the following represents the correct IUPAC name for the compounds concerned?(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyloctane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- 1-ol or But-4-ol-yne. |
| Answer: (a) 2, 2-Demethylpentane (b) 2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7.(c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-1-ol. Lower locant for the principal functional group, i.e., alcohol. |
| 12.6. Draw formulas for the first five members of each homologous series beginning with the following compounds. (a) H—COOH (b) CH3COCH3 (c) H—CH=CH2 |
| (a) H-COOH CH3—COOH CH3CH2CH2—COOH (b) CH3COCH3 CH3CH=CH2 |
Commonly asked questions
12.2. Indicate the σ and π-bonds in the following molecules:
C6H6, C6H12, CH2Cl2, CH=C=CH2, CH3NO2, HCONHCH3
Single bond contains only one sigma bond and double bond contains one sigma and one pi bond.

- In this structure, nine single bonds and three double bonds are present. So, there are 12σ and 3π bonds present.
- In this structure, eighteen single bonds are present. So, there are only 18σ bonds present.
- In this structure, four single bonds are present. So, there are only 4σ bonds present.
- In this structure, four single bonds and two double bonds are present. So, there are 6σ and 2π bonds present.
- In this structure, five single bonds and one double bond are present. So, there are 6σ and 1π bonds present.
- In this structure, seven single bonds and one double bond are present. So, there are 8σ and 1π bonds present.
12.5.Which of the following represents the correct IUPAC name for the compounds concerned?(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyloctane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- 1-ol or But-4-ol-yne.
(a) 2, 2-Demethylpentane
(b) 2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7.
(c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-1-ol. Lower locant for the principal functional group, i.e., alcohol.
12.6. Draw formulas for the first five members of each homologous series beginning with the following compounds.
(a) H—COOH (b) CH3COCH3 (c) H—CH=CH2
(a) H-COOH
CH3—COOH
CH3CH2—COOH
CH3CH2CH2—COOH
CH3CH2CH2CH2—COOH
(b) CH3COCH3
CH3COCH2CH3
CH3COCH2CH2CH3
CH3COCH2CH2CH2CH3
CH3CO (CH3)4CH3
(c) H—CH=CH2
CH3CH=CH2
CH3CH2CH=CH2
CH3CH2CH2CH=CH2
CH3CH2CH2CH2CH=CH2
12.9. Which of the two: O2NC H2CH2O– or CH3CH2O– is expected to be more stable and why?
Nitroethoxide ion (O2NCH2CH2O–) is more stable than ethoxide ion (CH3CH2O–) due to -I effect of nitro group which decreases the negative charge of oxide ion of nitroethoxide ion leading to stability. In contrast, CH3CH2 has +I-effect. It, therefore, tends to intensify the -ve charge and hence destabilizes it.
12.13. Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles:

(a) Here, HO– acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species.
(b) Here, –CN acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species.
(c) CH3C+O acts as an electrophile as it is an electron deficient species.
12.14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– → CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl → (CH3)2ClC—CH3
(c) CH3CH2Br + HO– → CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr → (CH3)2CBrCH2CH2CH3 + H2O
(a) Nucleophilic substitution (b) Electrophilic addition
(c) Bimolecular elimination (d) Nucleophilic substitution with rearrangement.
12.15. What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?

(a) They are structural isomers. The given compounds have the same molecular formula but they differ in the position of the functional group (here ketone group: first one at C-3 and second one at C-2 positions).
(b) They are geometrical isomers. Compounds having the same molecular formula, the same constitution, and the sequence of covalent bonds, but with the different relative position of their atoms in space are called geometrical isomers.
(c) They are resonance contributors because they differ in the position of electrons but not atoms.
12.17. Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
Inductive effect: The inductive effect refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another. Atoms or groups which lose electron towards a carbon atom are said to have +I Effect. Examples of +I effect are (Electron releasing)
(CH3)2C—, (CH3)2CH—, CH3CH2— CH3— etc.
Those atoms or groups which draw electron away from a carbon atom are said to have -I Effect. Examples of -I effect are:
NO2, F, Cl, Br, I, OH etc.
Electromeric effect: The electromeric effect refers to the polarity produced in a multiple bonded compound as it is approached by a reagent.
The atom A has lost its share of electron pair and B has gained this share.
As a result A acquires a positive charge and B a negative charge. It is a temporary effect and takes place only in the presence of a reagent.
(a) –I effect as shown below:
As the number of halogen atoms decreases, the overall -I effect decreases and the acid strength decreases accordingly.
(b) +I effect as shown below:
As the number of alkyl groups increases, the +I effect increases and the acid strength decreases accordingly.

12.18. Give a brief description of the principles of the following techniques taking an example in each case: (a) Crystallisation (b) Distillation (c) Chromatography
(a) Crystallisation: In this process the impure solid is dissolved in the minimum volume of a suitable solvent. The soluble impurities pass into the solution while the insoluble ones left behind. The hot solution is then filtered and allowed to cool undisturbed till crystallisation is complete. The crystals are then separated from the mother liquor by filtration and dried.
Example: crystallisation of sugar.
(b) Distillation: The operation of distillation is employed for the purification of liquids from non-volatile impurities. The impure liquid is boiled in a flask and the vapours so formed are collected and condensed to give back pure liquid in another vessel. Simple organic liquids such as benzene toluene, xylene etc. can be purified.
(c) Chromatography: Chromatography is based on the principle of selective distribution of the components of a mixture between two phases, a stationary phase and a moving phase. The stationary phase can be a solid or liquid, while the moving phase is a liquid or a gas. When the stationary phase is solid the basis is adsorption and when it is a liquid the basis is partition. Chromatography is generally used for the separation of coloured substances such as plant pigments or dyestuffs.
12.19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Fractional crystallisation is the method that can be used to separate two compounds with different solubilities in a solvent S. A hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out while the more soluble remains in the solution. The crystals are separated from the mother liquor and the mother liquor is again concentrated and the hot solution again allowed cooling when the crystals of the second compound are obtained. These are again filtered and dried.
12.20. What is the difference between distillation, distillation under reduced pressure and steam distillation? (Intermediate)
Distillation is used in case of volatile liquid mixed with non-volatile impurities.
Distillation under reduced pressure: This method is used to purify such liquids which have very high boiling points and which decompose at or below their boiling points.
Steam distillation is used to purify steam volatile liquids associated with water immiscible impurities.
12.22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method.
(i) Dumas method: The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water
(ii)Kjeldahl's method: A known mass of the organic compound is heated strongly with conc. H2SO4, a little potassium sulphate and a little mercury (a catalyst). As a result, the nitrogen present in the organic compound is converted to ammonium sulphate.
12.23. Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Estimation of halogens: It involves oxidising the organic substance with fuming nitric acid in the presence of silver nitrate. The halogen of the substance is thus converted to silver halide which is separated and weighed:
Weight of organic compound = W gm
weight of silver halide = x g.
% of halogen =
Estimation of sulphur: The organic substance is heated with fuming nitric acid but no silver nitrate is added. The sulphur of the substance is oxidised to sulphuric acid which is then precipitated as barium sulphate by adding excess of barium chloride solution. From the weight of BaSO4 so obtained the percentage of sulphur can be calculated.
% of sulphur = x
Estimation of phosphorous: The organic substance is heated with fuming nitric acid whereupon phosphorous is oxidised to phosphoric acid. The phosphoric acid is precipitated as ammonium phosphomolybdate, (NH4)3PO4.12MoO3, by the addition of ammonia and ammonium molybdate solution which is then separated, dried and weighed.
% of phosphorus =,
Where, molar mass of (NH4)3PO4.12MoO3 = 1877 g.
If phosphorus is estimated as Mg2P2O7
% of P = ![]()
12.26. Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Organic compound is fused with sodium metal so as to convert organic compounds into NaCN, Na2S, NaX and Na3PO4. Since these are ionic compounds and become more reactive and thus can be easily tested by suitable reagents.
12.27. Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Sublimation can be used for the separation of the two compounds because camphor can sublime whereas CaSO4 does not.
12.28. Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation?
It is because in steam distillation the sum of vapour pressure of organic compound and steam should be equal to atmospheric pressure.
12.29. Will CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer. (Intermediate)
No. CCl4 is a completely non-polar covalent compound whereas AgNO3 is ionic in nature. Therefore, they are not expected to react and thus a white precipitate of silver chloride will not be formed.
12.30. Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound?
CO2 is acidic in nature and therefore, it reacts with the strong base KOH to form K2CO3.
2KOH + CO2 ? K2CO3+ H2O.
12.31. Why is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
It necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test because sulphuric acid will react with lead acetate to form a white precipitate of lead sulphate which will interfere in the test of sulphur.
Pb (OCOCH3)2 + H2SO4 →PbSO4 + 2CH3COOH
12.32. An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0.20 g of this compound is subjected to complete combustion.
Step 1: Calculation of mass of CO2 produced
Mass of compound = 0.20 g
% of carbon = 69%
i.e. 12/44 x = Mass of Carbondioxide formed / Mass of Compound = 69/100
Therefore, mass of CO2formed = (69 x 44 x 0.20) / (12 x 100) = 0.506 g
Step 2: Calculation of mass of H2O produced
Mass of compound = 0.20 g
% of hydrogen = 4.8%
i.e. 2/18 x Mass of Water Formed/Mass of Compound = 4.8/100
Therefore, mass of H2O formed = 4.8*18*0.20/2*100 = 0.0864 g
12.33. A sample of 0.50 g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50 ml of 0.5M H2SO4. The residual acid required 60 ml of 0.5 M solution of NaOH for neutralization. Find the percentage composition of nitrogen in the compound.
Step I: Calculation of volume of unused acid i.e. V2 =?
V1 = Volume of NAOH solution required = 60 cm3
N1 = Normality of NaOH solution = ½ N
N2 = Normality of H2SO4 = 1N
Applying N1V1 = N2V2
½ N x 60 cm3 = 1N x V2
Or V2 = 30 cm3
Step II: calculation of volume of acid used
Volume of acid added = 50 cm3
Volume of unused acid = 30 cm3
Volume of acid used = 50 – 30 = 20 cm3
Step III: Calculation of % of nitrogen
Mass of compound = 0.50 g
Volume of acid used = 20 cm3
Normality of acid used = 1 N
% of nitrogen = (1.4 x 20 x 1) / 0.50 = 56%
12.34. 0.3780 g of an organic compound gave 0.5740 g of silver chloride in Carius estimation. Calculate the percentage of chlorine present in the compound.
Mass of the compound = 0.3780 g
Mass of silver chloride = 0.5740 g
% of chlorine =![]()

12.35. In an estimation of sulphur by Carius method, 0.468 of an organic sulphur compound gave 0.668 g of barium sulphate. Find out the percentage of sulphur in the compound.
Mass of the compound = 0.468 g
Mass of barium sulphate= 0.668 g
% of sulphur = ![]()

12.37. In Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of:
(a) Na4[Fe(CN)6] (b)Fe4[Fe(CN)6]3
(c) Fe2[Fe(CN)6] (d)Fe3[Fe(CN)6]4 . (Intermediate)
(b) Iron (III) hexacyanidoferrate (II) (or ferriferrocyanide) Fe4 [Fe (CN)6]3 is the correct answer.
12.39. The best and latest technique for isolation, purification and separation of organic compounds is:
(a) Crystallisation (b) Distillation(c) Sublimation (d) Chromatography
(d) Is the correct answer.
12.40. The reaction:
CH3CH2I + KOH (aq) → CH3CH2OH + KI is classified as:
(a) electrophilic substitution (b) nucleophilic substitution
(c) elimination (d) addition
(b) It is a nucleophilic substitution reaction. KOH (aq) provides OH- ion for the nucleophile attack.
12.43.Assertion: In homolytic cleavage, the movement of a single electron takes place instead of an electron pair.
Reason: A homolytic cleavage yields carbocations or carbanions.
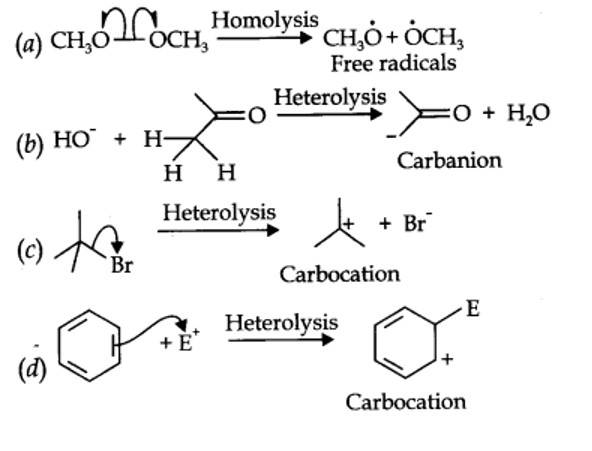
(c) In homolytic cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, in homolytic cleavage, the movement of a single electron takes place instead of an electron pair.
A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate.
12.44. Assertion: The energy of actual structure of the molecule(the resonance hybrid) is lower than that of anyof the canonical structures.
Reason: Resonance is particularly important when the contributing structures are equivalent in energy.
(b) The difference in energy between the actual structure and the lowest energy resonance structure is called the resonance stabilisation energy or simply the resonance energy. The more the number of important contributing structures, the more is the resonance energy.
12.5. Assertion: Electromeric effect is a permanent effect.
Reason: Organic compounds having a multiple bond show this effect in the presence of an attacking reagent.
(d) Electromeric effect is a temporary effect. The organic compounds having a multiple bond (a doubleor triple bond) show this effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of? -electrons to one of the atoms joined by multiple bond on the demand of an attacking reagent.
MCQs
12.46 Hyperconjugation is a
(a) permanent effect (b) temporary effect (c) dual effect (d) none
(a) Permanent effect
12.47. The common techniques used for purification are as follows:
(a) Sublimation
(b) Crystallisation
(c) Distillation
(d) all of the above
(d) All of the above
12.3. Nitrogen is estimated by
(a) Carius method (b) Dumas method(c) Kjeldahl’s method (d) both b and c
(d) Both b and c
12.49. Which elements are estimated by Liebig’s Method?
(a) Oxygen and nitrogen (b) Carbon and hydrogen (c) Sulphur and Phosphorus (d) Calcium and potassium
(b) Carbon and hydrogen
12.50. A nucleophile
(a) Brings an electron pair to thereactive site
(b) Takes away anelectron pair from reactive site
(c) Brings nucleus of one atom near the nucleus of another atom
(d) Brings electrons of one atom near the electrons of another atom
(a) Brings an electron pair to the reactive site
12.5. Name the methods for separation of the following:
(i) mixture of two organic compounds having different solubilities in the same solvent
(ii) naphthalene from kerosene oil
(iii) organic liquid that decomposes below its boiling point
(i) By fractional crystallisation
(ii) Simple distillation
(iii Distillation under reduced pressure.
12.4. Give the IUPAC names of the following compounds

(a) Propylbenzene (b) 3-Methylpentanenitrite (c) 2, 5-Dimethylheptane
(d) 3-Bromo- 3-chloroheptane (e) 3-Chloropropanal (f) 2, 2-Dichloroethanol
12.10. Explain why alkyl groups act as electron donors when attached to a π-system.
Due to hyperconjugation, alkyl groups act as electron donors when attached to a π - system as shown below:

12.12. What are electrophiles and nucleophiles? Explain with examples.
Electrophiles: The name electrophiles mean electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules.
Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3
Nucleophiles: The name nucleophiles means 'nucleus loving' and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule. They are electron rich they may be negative ions or neutral molecules.
Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
12.21. Discuss the chemistry of Lassaigne’s test.
Lassaigne's test: In organic compounds, nitrogen, sulphur and halogens are covalently bonded. Their detection in 'Lassaigne's test' is possible if they are in the ionic form. This can be achieved by fusing the organic compound with sodium metal.
Chemistry for test for nitrogen:
Sodium fusion extract is boiled with ferrous sulphate and acidified with sulphuric acid. Sodium cyanide reacts with ferrous sulphate and forms sodium hexacyanoferrate (II). On heating with sulphuric acid, some ferrous is oxidized to ferric hexacyanoferate (II) Fe4 [Fe (CN)6]3 which is prussian blue in colour.
Chemistry of the test for sulphur:
Acetic acid is added to sodium fusion extract. Complete precipitation of sulphur in the form of lead sulphate occurs which is black in colour. Also, the sodium fusion extract is treated with sodium nitroprusside to obtain violet color. However if N and S are present, then instead of NaCN, NaSCN is obtained. This gives blood red colour on reaction with Fe3+ ions.
Chemistry of test for halogens:
Sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. However, if nitrogen and sulphur both are present, then Lassaigne's extract is boiled to expel nitrogen and sulphur which would otherwise interfere in the test for halogens.
12.24. Explain the principle of paper chromatography.
This is the simplest form of chromatography. Here a strip of paper acts as an adsorbent. It is based on the principle which is partly adsorption. The paper is made of cellulose fibres with molecules of water adsorbed on them. This acts as stationary phase. The mobile phase is the mixture of the components to be identified prepared in a suitable solvent.
12.25. Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
Nitric acid is added to sodium extract so as to decompose NaCN to HCN and Na2S to H2S and to expel these gases.
NaCN + HNO3 ——-> NaNO3 + HCN
Na2S + 2HNO3 ——> 2NaNO3 + H2S
12.38. Which of the following carbocation is most stable? (Intermediate)

(b) Is the most stable since it is a tertiary carbocation.
Questions and Answers
12.51.Explain differential extraction.
When an organic compound is present in anaqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water. The organic solvent and the aqueous solution should be immiscible with each other so that they form two distinct layers which can be separated by separatory funnel. The organic solvent is later removed by distillation or by evaporation to get backthe compound. Differential extraction is carried out in a separatory funnel.
If the organic compound is less soluble in the organic solvent, a very large quantity of solvent would be required to extract even a very small quantity of the compound. The technique of continuous extraction is employed in such cases. In this technique same solvent is repeatedly used for extraction of the compound.
12.52. What is Buckminsterfullerene?
Buckminsterfullerene is a common name given to the newly discovered C60 cluster (a form of carbon) noting its structural similarity to the geodesic domes popularised by the famous architect R. Buckminster Fuller.
12.3 Define resonance effect.
The resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π -bond and lone pair of electrons present on an adjacent atom'. The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as R or M effect.
12.54. Mention one difference between a homolytic and a heterolytic cleavage.
A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate.
12.6. Explain is steam distillation.
Steam distillation is applied to separate substances which are steam volatile and are immiscible with water. In steam distillation, steam from a steam generator is passed through a heated flask containing the liquid to be distilled. The mixture of steam and the volatile organic compound is condensed and collected. The compound is later separated from water using a separating funnel. In steam distillation, the liquid boils when the sum of vapour pressures due to the organic liquid (p1) and that due to water (p2) becomes equal to the atmospheric pressure (p), i.e. p =p1+ p2. Since p1 is lower than p, the organic liquid vaporises at lower temperature than its boiling point.
12.57. Explain hyperconjugation effect. Is hyperconjugation a temporary effect?
Hyperconjugation is a general stabilising interaction. It involves delocalisation of σ electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The σ electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Greater the hyperconjugation, greater will be the stability of alkenes.
No, hyperconjugation is not a temporary effect. It is a permanent effect.
12.58. What do you understand by conjugated system in organic chemistry?
The presence of alternate single and double bonds in an open chain or cyclic system is termed as a conjugated system. These systems often show abnormal behaviour. The examples are 1, 3- butadiene, aniline and nitrobenzene etc. In such systems, the π -electrons are delocalised and the system develops polarity.
12.59. What do you understand by resonance effect? Name the two types of it.
The resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π -bonds or between a π -bond and lone pair of electrons present on an adjacent atom'. The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as +R and-R effect.
The atoms or substituent groups, whichrepresent +R or –R electron displacementeffects are as follows:
+R effect: – halogen, –OH, –OR, –OCOR, –NH2, –NHR, –NR2, –NHCOR,
– R effect: – COOH, –CHO, >C=O, – CN, –NO2
12.60. Mention the rules to be applied while writing resonance structures.
The resonance structures have
(i) The same positions of nuclei, and
(ii) The same number ofunpaired electrons.
12.1. What are hybridisation states of each carbon atom in the following compounds? CH2=C=O, CH3CH=CH2, (CH3)2CO, CH2=CHCN, C6H6.
Kindly go through the solution
12.3. Write bond-line formulas for: Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one.
Kindly go through the solution
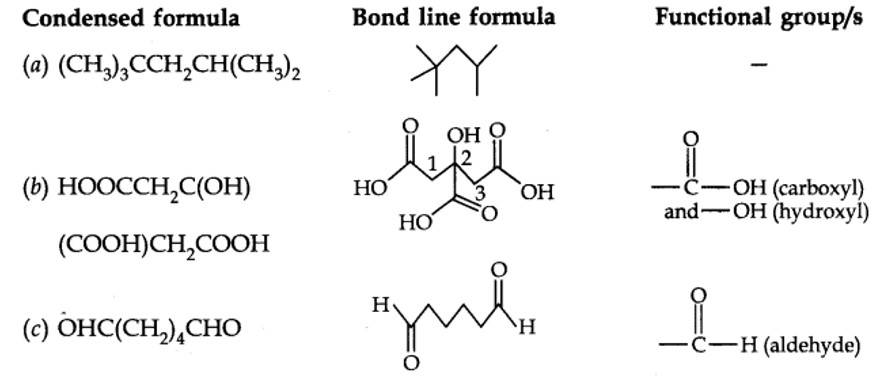
12.7. Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for:
(a) 2, 2, 4-Trimethylpentane
(b) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid
(c) Hexanedial
Kindly go through the solution
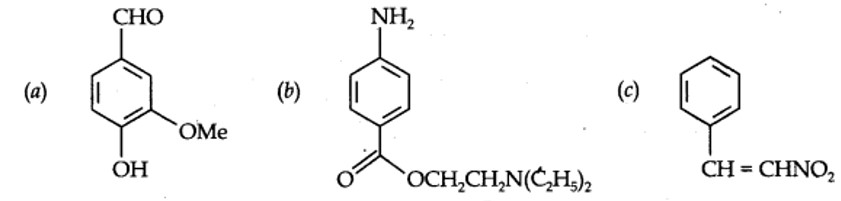
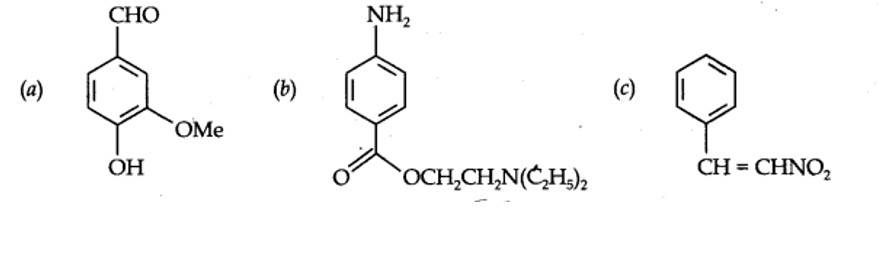
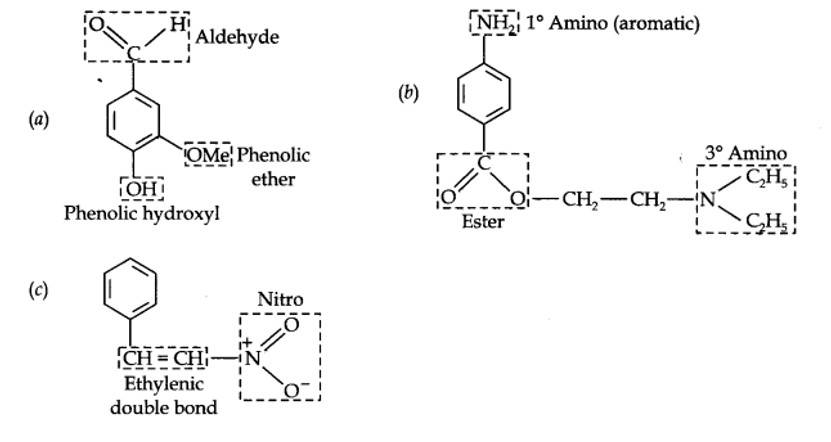
Identify the functional groups in the following compounds
Kindly go through the solution
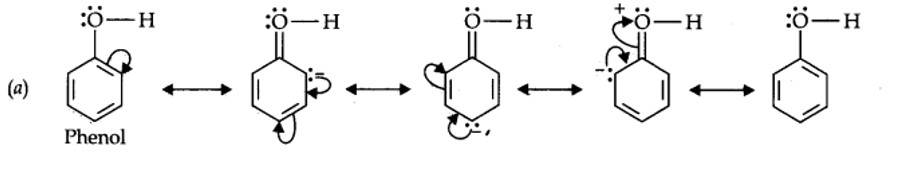
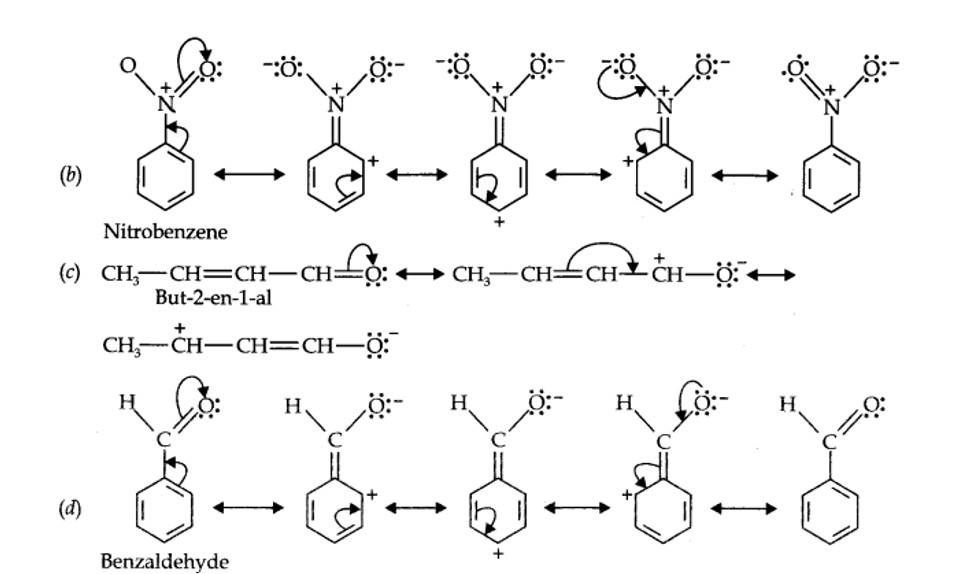
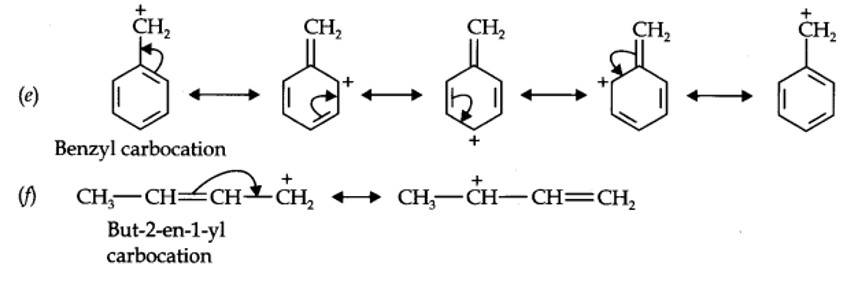
12.11. Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C6H5OH (b) C6H5NO2 (c) CH3CH=CHCHO (d) C6H5—CHO (e) C6H5—CH2 (f) CH3CH=CHCH2
Kindly go through the solution
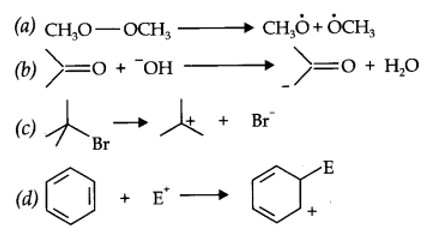
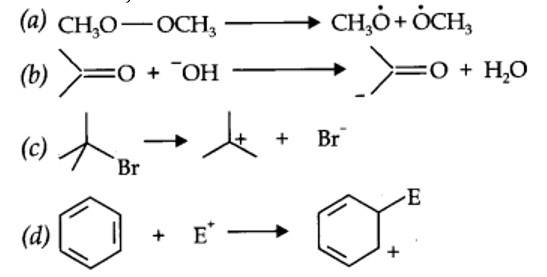
12.16. For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
Kindly go through the solution
12.36. In the organic compound CH2 = CH – CH2 – CH2 – C ≡ CH, the pair of hybridised orbitals involved in the formation of: C2 – C3 bond is:
(a) sp – sp2 (b) sp – sp3 (c) sp2 – sp3 (d) sp3 – sp3
Kindly go through the solution
(c) sp2 – sp3
12.41. Assertion: In general, π bonds provide the most reactive centres in the molecules containingmultiple bonds.
Reason: The electron charge cloud of the π bond is located above and below the plane of bonding atoms. The electrons being easily available to the attacking reagents.
Kindly go through the solution
(a)
12.2. Assertion: Citric acid is namedso because it is found in citrus fruits.
Reason: Prior to IUPAC system of nomenclature, organic compounds were assigned
names based on their origin or other properties.
Kindly go through the solution
(a)
Organic Chemistry - Some Basic Principles and Techniques Structure More Solutions
Class 11 Organic Chemistry - Some Basic Principles and Techniques NCERT extra solutions are provided below. These questions are based on types of organic reactions, purification of organic compounds, nature of organic reactions, organic reaction mechanisms, qualitative analysis of organic compounds, organic chemistry practical techniques, etc. Students whose basic concepts on these topics are not clear must check the NCERT chemistry book chapter explanations to understand the topics in a better way.
| 12.5.Which of the following represents the correct IUPAC name for the compounds concerned?(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyloctane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- 1-ol or But-4-ol-yne. |
| Answer: (a) 2, 2-Demethylpentane (b) 2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7.(c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-1-ol. Lower locant for the principal functional group, i.e., alcohol. |
| 12.6. Draw formulas for the first five members of each homologous series beginning with the following compounds. (a) H—COOH (b) CH3COCH3 (c) H—CH=CH2 |
| (a) H-COOH CH3—COOH CH3CH2CH2—COOH (b) CH3COCH3 CH3CH=CH2 CH3CH2CH=CH2 |
| 12.7. Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for: (a) 2, 2, 4-Trimethylpentane (b) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid (c) Hexanedial |
| Answer: |
| 12.8. Identify the functional groups in the following compounds
|
| Answer:
|
| 12.9. Which of the two: O2NC H2CH2O– or CH3CH2O– is expected to be more stable and why? |
| Answer: Nitroethoxide ion (O2NCH2CH2O–) is more stable than ethoxide ion (CH3CH2O–) due to -I effect of nitro group which decreases the negative charge of oxide ion of nitroethoxide ion leading to stability. In contrast, CH3CH2 has +I-effect. It, therefore, tends to intensify the -ve charge and hence destabilizes it. |
| 12.10. Explain why alkyl groups act as electron donors when attached to a π-system. |
| Answer: Due to hyperconjugation, alkyl groups act as electron donors when attached to a π- system as shown below:
|
| 12.11. Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C6H5OH (b) C6H5NO2 (c) CH3CH=CHCHO (d) C6H5—CHO (e) C6H5—CH2 (f) CH3CH=CHCH2 |
| Answer: |
| 12.12. What are electrophiles and nucleophiles? Explain with examples. |
| Answer: Electrophiles: The name electrophiles mean electron loving. Electrophiles are electron deficient. They may be positive ions or neutral molecules. Ex: H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3 Nucleophiles: The name nucleophiles means ‘nucleus loving’ and indicates that it attacks the region of low electron density (positive centres) in a substrate molecule. They are electron rich they may be negative ions or neutral molecules. Ex: Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc. |
| 12.13. Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles:
|
| Answer: (a) Here, HO– acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species. (b) Here, –CN acts as a nucleophile as it is an electron-rich species, i.e., it is a nucleus-seeking species. (c) CH3C+O acts as an electrophile as it is an electron deficient species.
|
| 12.14. Classify the following reactions in one of the reaction type studied in this unit. (a) CH3CH2Br + HS– → CH3CH2SH + Br– (b) (CH3)2C=CH2 + HCl → (CH3)2ClC—CH3 (c) CH3CH2Br + HO– → CH2=CH2 + H2O + Br– (d) (CH3)3C—CH2OH + HBr → (CH3)2CBrCH2CH2CH3 + H2O |
| Answer: (a) Nucleophilic substitution (b) Electrophilic addition (c)Bimolecular elimination (d) Nucleophilic substitution with rearrangement. |
| 12.15. What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors? |
| Answer: (a) They are structural isomers. The given compounds have the same molecular formula but they differ in the position of the functional group (here ketone group: first one at C-3 and second one at C-2 positions). |
| 12.16. For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion. |
| Answer: |
| 12.17. Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids? (a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH (b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH |
| Answer: Inductive effect: The inductive effect refers to the polarity produced in a molecule as a result of higher electronegativity of one atom compared to another. Atoms or groups which lose electron towards a carbon atom are said to have +I Effect. Examples of +I effect are (Electron releasing) (CH3)2C—, (CH3)2CH—, CH3CH2— CH3— etc. Those atoms or groups which draw electron away from a carbon atom are said to have -I Effect. Examples of -I effect are: NO2, F, Cl, Br, I, OH etc. Electromeric effect: The electromeric effect refers to the polarity produced in a multiple bonded compound as it is approached by a reagent. The atom A has lost its share of electron pair and B has gained this share. As a result A acquires a positive charge and B a negative charge. It is a temporary effect and takes place only in the presence of a reagent. (a) –I effect as shown below: As the number of halogen atoms decreases, the overall -I effect decreases and the acid strength decreases accordingly. (b) +I effect as shown below: As the number of alkyl groups increases, the +I effect increases and the acid strength decreases accordingly. |
| 12.18. Give a brief description of the principles of the following techniques taking an example in each case: (a) Crystallisation (b) Distillation (c) Chromatography |
| Answer: (a) Crystallisation: In this process the impure solid is dissolved in the minimum volume of a suitable solvent. The soluble impurities pass into the solution while the insoluble ones left behind. The hot solution is then filtered and allowed to cool undisturbed till crystallisation is complete. The crystals are then separated from the mother liquor by filtration and dried. |
| 12.19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S. |
| Answer: Fractional crystallisation is the method that can be used to separate two compounds with different solubilities in a solvent S. A hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out while the more soluble remains in the solution. The crystals are separated from the mother liquor and the mother liquor is again concentrated and the hot solution again allowed cooling when the crystals of the second compound are obtained. These are again filtered and dried. |
| 12.20. What is the difference between distillation, distillation under reduced pressure and steam distillation? (Intermediate) |
| Answer: Distillation is used in case of volatile liquid mixed with non-volatile impurities. |
| 12.21. Discuss the chemistry of Lassaigne’s test. |
| Answer: Lassaigne’s test: In organic compounds, nitrogen, sulphur and halogens are covalently bonded. Their detection in 'Lassaigne's test' is possible if they are in the ionic form. This can be achieved by fusing the organic compound with sodium metal. Chemistry for test for nitrogen: Sodium fusion extract is boiled with ferrous sulphate and acidified with sulphuric acid. Sodium cyanide reacts with ferrous sulphate and forms sodium hexacyanoferrate(II). On heating with sulphuric acid, some ferrous is oxidized to ferric hexacyanoferate (II) Fe4[Fe(CN)6]3 which is prussian blue in colour. Chemistry of the test for sulphur: Acetic acid is added to sodium fusion extract. Complete precipitation of sulphur in the form of lead sulphate occurs which is black in colour. Also, the sodium fusion extract is treated with sodium nitroprusside to obtain violet color. However if N and S are present, then instead of NaCN, NaSCN is obtained. This gives blood red colour on reaction with Fe3+ ions. Chemistry of test for halogens: Sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. However, if nitrogen and sulphur both are present, then Lassaigne's extract is boiled to expel nitrogen and sulphur which would otherwise interfere in the test for halogens. |
| 12.22. Differentiate between the principle of estimation of nitrogen in an organic compound by (i) Dumas method (ii) Kjeldahl’s method. |
| Answer: (i) Dumas method: The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields free nitrogen in addition to carbon dioxide and water (ii)Kjeldahl’s method: A known mass of the organic compound is heated strongly with conc. H2SO4, a little potassium sulphate and a little mercury (a catalyst). As a result, the nitrogen present in the organic compound is converted to ammonium sulphate. |
| 12.23. Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound. |
| in an organic compound. % of halogen = Estimation of sulphur: The organic substance is heated with fuming nitric acid but no silver nitrate is added. The sulphur of the substance is oxidised to sulphuric acid which is then precipitated as barium sulphate by adding excess of barium chloride solution. From the weight of BaSO4 so obtained the percentage of sulphur can be calculated. % of sulphur = x % of phosphorus = , Where, molar mass of (NH4)3PO4.12MoO3 = 1877 g. If phosphorus is estimated as Mg2P2O7 % of P = |
| 12.24. Explain the principle of paper chromatography. |
| Answer: This is the simplest form of chromatography. Here a strip of paper acts as an adsorbent. It is based on the principle which is partly adsorption. The paper is made of cellulose fibres with molecules of water adsorbed on them. This acts as stationary phase. The mobile phase is the mixture of the components to be identified prepared in a suitable solvent. |
| 12.25. Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens? |
| Answer: Nitric acid is added to sodium extract so as to decompose NaCN to HCN and Na2S to H2S and to expel these gases. NaCN + HNO3 ——-> NaNO3 + HCN |
| 12.26. Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens. |
| Answer: Organic compound is fused with sodium metal so as to convert organic compounds into NaCN, Na2S, NaX and Na3PO4. Since these are ionic compounds and become more reactive and thus can be easily tested by suitable reagents. |
| 12.27. Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor. |
| Answer: Sublimation can be used for the separation of the two compounds because camphor can sublime whereas CaSO4 does not. |
| 12.28. Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation? |
| Answer: It is because in steam distillation the sum of vapour pressure of organic compound and steam should be equal to atmospheric pressure. |
| 12.29. Will CCl4 give white precipitate of AgCl on heating it with silver nitrate? Give reason for your answer. (Intermediate) |
| Answer: No. CCl4 is a completely non-polar covalent compound whereas AgNO3 is ionic in nature. Therefore, they are not expected to react and thus a white precipitate of silver chloride will not be formed. |
| 12.30. Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound? |
| Answer: CO2 is acidic in nature and therefore, it reacts with the strong base KOH to form K2CO3. |
| 12.31. Why is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test? |
| Answer: It necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test because sulphuric acid will react with lead acetate to form a white precipitate of lead sulphate which will interfere in the test of sulphur. Pb(OCOCH3)2 + H2SO4 → PbSO4 + 2CH3COOH |
| 12.32. An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the masses of carbon dioxide and water produced when 0.20 g of this compound is subjected to complete combustion. |
| Answer: Step 1: Calculation of mass of CO2 produced Mass of compound = 0.20 g % of carbon = 69% i.e. 12/44 x = Mass of Carbondioxide formed / Mass of Compound = 69/100 Therefore, mass of CO2formed = (69 x 44 x 0.20) / (12 x 100) = 0.506 g Step 2: Calculation of mass of H2O produced Mass of compound = 0.20 g % of hydrogen = 4.8% i.e. 2/18 x Mass of Water Formed/Mass of Compound = 4.8/100 Therefore, mass of H2O formed = 4.8*18*0.20/2*100 = 0.0864 g |
| 12.33. A sample of 0.50 g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50 ml of 0.5M H2SO4. The residual acid required 60 ml of 0.5 M solution of NaOH for neutralization. Find the percentage composition of nitrogen in the compound. |
| Answer: Step I: Calculation of volume of unused acid i.e. V2 = ? V1 = Volume of NAOH solution required = 60 cm3 N1 = Normality of NaOH solution = ½ N N2 = Normality of H2SO4 = 1N Applying N1V1 = N2V2 ½ N x 60 cm3 = 1N x V2 Or V2 = 30 cm3 Step II: calculation of volume of acid used Volume of acid added = 50 cm3 Volume of unused acid = 30 cm3 Volume of acid used = 50 – 30 = 20 cm3 Step III: Calculation of % of nitrogen Mass of compound = 0.50 g Volume of acid used = 20 cm3 Normality of acid used = 1 N % of nitrogen = (1.4 x 20 x 1) / 0.50 = 56% |
| 12.34. 0.3780 g of an organic compound gave 0.5740 g of silver chloride in Carius estimation. Calculate the percentage of chlorine present in the compound. |
| Answer: Mass of the compound = 0.3780 g % of chlorine = |
| 12.35. In an estimation of sulphur by Carius method, 0.468 of an organic sulphur compound gave 0.668 g of barium sulphate. Find out the percentage of sulphur in the compound. |
| Answer: Mass of the compound = 0.468 g % of sulphur = |
| 12.36. In the organic compound CH2 = CH – CH2 – CH2 – C ≡ CH, the pair of hybridised orbitals involved in the formation of: C2 – C3 bond is: (a) sp – sp2 (b) sp – sp3 (c) sp2 – sp3 (d) sp3 – sp3 |
| Answer:(c) sp2 – sp3 |
| 12.37. In Lassaigne’s test for nitrogen in an organic compound, the Prussian blue colour is obtained due to the formation of: (a) Na4[Fe(CN)6] (b)Fe4[Fe(CN)6]3 (c) Fe2[Fe(CN)6] (d)Fe3[Fe(CN)6]4 . (Intermediate) |
| Answer: (b) iron(III) hexacyanidoferrate(II) (or ferriferrocyanide) Fe4[Fe(CN)6]3 is the correct answer. |
| 12.38. Which of the following carbocation is most stable? (Intermediate) |
| Answer: (b) is the most stable since it is a tertiary carbocation. |
| 12.39. The best and latest technique for isolation, purification and separation of organic compounds is: (a) Crystallisation (b) Distillation(c) Sublimation (d) Chromatography |
| Answer: (d) is the correct answer. |
| 12.40. The reaction: (a) electrophilic substitution (b) nucleophilic substitution (c) elimination (d) addition |
| Answer: (b) It is a nucleophilic substitution reaction. KOH (aq) provides OH- ion for the nucleophile attack. |
Organic Chemistry - Some Basic Principles and Techniques Structure Additional Questions
Students who wish to challenge their knowledge on the NCERT Class 11 Chemistry Chapter 8 Organic Chemistry – Some Basic Principles and Techniques can solve these brainstorming questions.
| Assertion and Reason: Directions: (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement. 12.1. Assertion: In general, π bonds provide the most reactive centres in molecules containing multiple bonds. Reason: The electron charge cloud of the π bond is located above and below the plane of bonding atoms. The electrons being easily available to the attacking reagents. |
| Answer:(a) |
| 12.2. Assertion: Citric acid is namedso because it is found in citrus fruits. Reason: Prior to IUPAC system of nomenclature, organic compounds were assigned names based on their origin or other properties. |
| Answer: (a) |
| 12.3.Assertion: In homolytic cleavage, the movement of a single electron takes place instead of an electron pair. Reason: A homolytic cleavage yields carbocations or carbanions. |
| Answer: (c) In homolytic cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, in homolytic cleavage, the movement of a single electron takes place instead of an electron pair. A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate. |
| 12.4. Assertion: The energy of actual structure of the molecule(the resonance hybrid) is lower than that of anyof the canonical structures. Reason: Resonance is particularly important when the contributing structures are equivalent in energy. |
| Answer: (b) The difference in energy between the actual structure and the lowest energy resonance structure is called the resonance stabilisation energy or simply the resonance energy. The more the number of important contributing structures, the more is the resonance energy. |
| 12.5. Assertion: Electromeric effect is a permanent effect. Reason: Organic compounds having a multiple bond show this effect in the presence of an attacking reagent. |
| Answer: (d) Electromeric effect is a temporary effect. The organic compounds having a multiple bond (a doubleor triple bond) show this effect in the presence of an attacking reagent only. It is defined as the complete transfer of a shared pair of π-electrons to one of the atoms joined by multiple bond on the demand of an attacking reagent. |
| MCQs 12.1 Hyperconjugation is a (a) permanent effect (b) temporary effect (c) dual effect (d) none |
| Answer: (a)permanent effect |
| 12.2. The common techniques used for purification are as follows: (a) Sublimation (b) Crystallisation (c) Distillation (d) all of the above |
| Answer: (d) all of the above |
| 12.3. Nitrogen is estimated by (a) Carius method (b) Dumas method(c) Kjeldahl’s method (d) both b and c |
| Answer: (d) both b and c |
| 12.4. Which elements are estimated by Liebig’s Method? (a) oxygen and nitrogen (b) Carbon and hydrogen (c) Sulphur and Phosphorus (d) Calcium and potassium |
| Answer: (b) Carbon and hydrogen |
| 12.5. A nucleophile (a) brings an electron pair to thereactive site (b) takes away anelectron pair from reactive site (c) brings nucleus of one atom near the nucleus of another atom (d) brings electrons of one atom near the electrons of another atom |
| Answer: (a) brings an electron pair to the reactive site |
| Questions and Answers 12.1.Explain differential extraction. |
| Answer: When an organic compound is present in anaqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water. The organic solvent and the aqueous solution should be immiscible with each other so that they form two distinct layers which can be separated by separatory funnel. The organic solvent is later removed by distillation or by evaporation to get backthe compound. Differential extraction is carried out in a separatory funnel. If the organic compound is less soluble in the organic solvent, a very large quantity of solvent would be required to extract even a very small quantity of the compound. The technique of continuous extraction is employed in such cases. In this technique same solvent is repeatedly used for extraction of the compound. |
| 12.2. What is Buckminsterfullerene? |
| Answer: Buckminsterfullerene is a common name given to the newly discovered C60 cluster (a form of carbon) noting its structural similarity to the geodesic domes popularised by the famous architect R. Buckminster Fuller. |
| 12.3 Define resonance effect. |
| Answer: The resonance effect is defined as ‘the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacent atom’. The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as R or M effect. |
| 12.4. Mention one difference between a homolytic and a heterolytic cleavage. |
| Answer: A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate. |
| 12.5. Name the methods for separation of the following: (i) mixture of two organic compounds having different solubilities in the same solvent (iii) organic liquid that decomposes below its boiling point |
| Answer: (i) By fractional crystallisation (ii) Simple distillation (iii) distillation under reduced pressure. |
| 12.6. Explain is steam distillation. |
| Answer: Steam distillation is applied to separate substances which are steam volatile and are immiscible with water. In steam distillation, steam from a steam generator is passed through a heated flask containing the liquid to be distilled. The mixture of steam and the volatile organic compound is condensed and collected. The compound is later separated from water using a separating funnel. In steam distillation, the liquid boils when the sum of vapour pressures due to the organic liquid (p1) and that due to water (p2) becomes equal to the atmospheric pressure (p), i.e. p =p1+ p2. Since p1 is lower than p, the organic liquid vaporises at lower temperature than its boiling point. |
| 12.7. Explain hyperconjugation effect. Is hyperconjugation a temporary effect? |
| Answer: Hyperconjugation is a general stabilising interaction. It involves delocalisation of σ electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The σ electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Greater the hyperconjugation, greater will be the stability of alkenes. No, hyperconjugation is not a temporary effect. It is a permanent effect. |
| 12.8. What do you understand by conjugated system in organic chemistry? |
| Answer: The presence of alternate single and double bonds in an open chain or cyclic system is termed as a conjugated system. These systems often show abnormal behaviour. The examples are 1, 3- butadiene, aniline and nitrobenzene etc. In such systems, the π-electrons are delocalised and the system develops polarity. |
| 12.9. What do you understand by resonance effect? Name the two types of it. |
| Answer: The resonance effect is defined as ‘the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacent atom’. The effect is transmitted through the chain. There are two types of resonance or mesomeric effect designated as +R and-R effect. The atoms or substituent groups, whichrepresent +R or –R electron displacementeffects are as follows: +R effect: – halogen, –OH, –OR, –OCOR, –NH2,–NHR, –NR2, –NHCOR, – R effect: – COOH, –CHO, >C=O, – CN, –NO2 |
| 12.10. Mention the rules to be applied while writing resonance structures. |
| Answer: The resonance structures have (i) the same positions of nuclei, and (ii) (ii) the same number ofunpaired electrons. |
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test