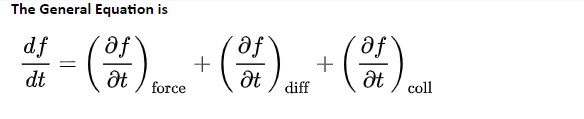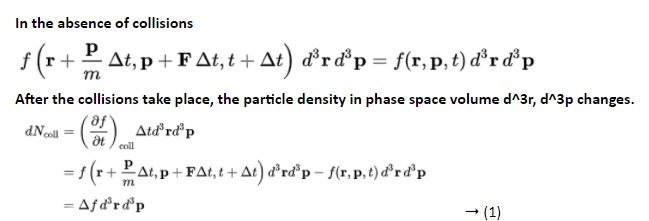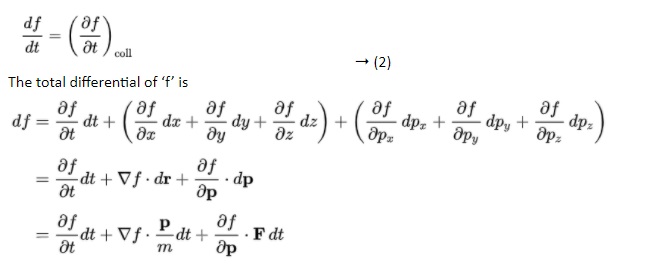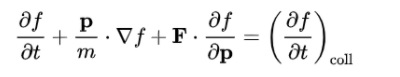- Introduction
- Boltzmann Equation
- Distribution Functions
- The Boltzmann Equation for Class 11
- FAQs on Boltzmann Equation
Introduction
If we take a hot, dense gas, the large numbers of atoms collide with each other, leading to the excitation to the various energy levels. After some time scales, molecules exhibit radiative de-excitation. At constant temperature and pressure, equilibrium built between the collisional excitations and radiative deexcitations.
- The equilibrium leads to the distribution of atoms between the energy levels. At higher temperatures, the large number of atoms occupy at high energy levels.
- Thus the Boltzmann equation shows the distribution of atoms among different energy levels as a function of energy and temperature.
Boltzmann Equation
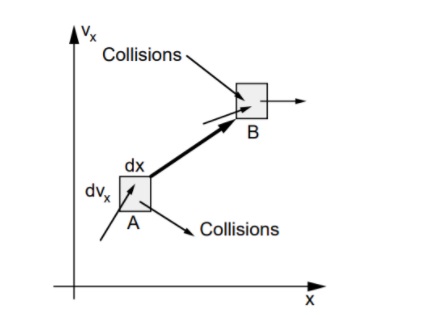
The Boltzmann equation helps us to define the properties of dilute gases. It analyses the elementary collision processes between a pair of molecules. In general, the Boltzmann equation is expressed as a kinetic equation that describes the change of macroscopic quantity in a thermodynamic system, such as energy, charge or particle number.
Distribution Functions
- A plasma is an ensemble of particles electrons ‘e,’ ions ‘i’ and neutrals ‘n’ with different positions ‘r’ and velocities ‘v,’ which move under the influence of external forces (electromagnetic fields, gravity) and internal collision processes (ionisation, Coulomb, charge exchange)
- The macroscopic plasma parameters are j - current density, ne - electron density, P - pressure, and Ti - ion temperature.
The Boltzmann Equation for Class 11
In chapter ‘Thermodynamics,’ the Boltzmann equation has less probability than other thermodynamics laws. The Boltzmann equation is an elaborated topic with different applications in physics. The weightage of this topic is below 5 marks.
FAQs on Boltzmann Equation
Q: What are the applications of the Boltzmann equation?
Q: What is the Maxwell-Boltzmann distribution?
Q: What is the Collisionless Boltzmann Equation?
Q: What is the convective derivative?
Q: What is the Boltzmann Planck equation?
Q: What are the macroscopic variables?
Physics Thermodynamics Exam
Student Forum
Other Topics under this Chapter
- Uses of Colorimeter
- Specific Heat Capacity
- Specific Heat
- Law of Conservation of Mass
- Law of Conservation of Energy
- Van Der Waals Equation
- Third law of thermodynamics
- Boltzmann Equation
- Helmholtz Equation
- Helmholtz Free Energy
- Maxwell Relations
- Chemical Thermodynamics
- Law of Equipartition of Energy
- Clausius Statement
- Carnot Theorem
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test