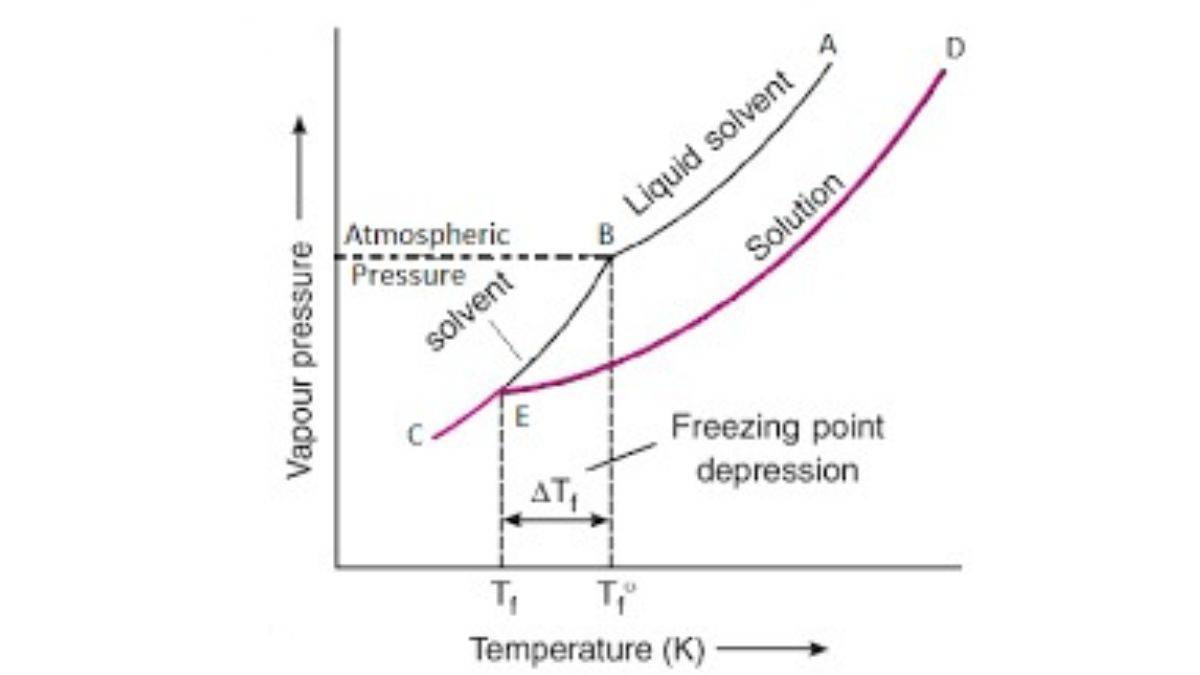
Depression in freezing point is a property of solutions observed when a non-volatile solid is added to the solvent, its vapour pressure decreases. This phenomenon is explained by Raoult's law. Freezing point depression has various applications such as de-icing roads, making Ice cream, food preservation, determining molar mass, etc.
Depression of freezing point is a topic in Class 12 Chemistry Chapter 1 Solutions. Students can learn about this topic through their textbook. Also, the experts at Shiksha have prepared an NCERT solution to help students prepare for the exam. CBSE Class 12 Chemistry Chapter 1 NCERT Solution is the best study material for self-assessment and preparation.
In this article, we will discuss what is depression in freezing point, depression of freezing point formulas, and the application of freezing point depression.
Also Check: NCERT Notes
- Defination of Depression in Freezing Point
- Depression in Freezing Point Formula
- Factors Affecting Depression in Freezing Point
- Freezing Point and Depression of Freezing Point for Solvents
- Uses of Freezing Point Depression
- FAQs on Depression in Freezing Point
Defination of Depression in Freezing Point
The depression in freezing point is a colligative property of a solution. It is also known as freezing point depression. Depression is referred to as an event where the freezing point of a solution is lower than that of the pure solvent. The reason for this difference is that the presence of solute particles disrupts the regular crystal lattice structure that forms when a pure solvent freezes. The formula representation of the depression of freezing point is
ΔTf = i×Kf×m
Depression in Freezing Point Formula
When a non-volatile solute is dissolved in the solvent, then the freezing point of the solution is lower than that of the pure solvent. This happens because a non-volatile solute decreases the vapour pressure of the liquid solution. A decrease in pressure lowers the freezing point. This lowering is called depression in freezing point.
Where:
- ΔTf is the change in freezing point (in degrees Celsius or Kelvin).
- Kf is the cryoscopic constant, which is a characteristic constant for a particular solvent.
- m is the molality of the solute, which is the moles of solute per kilogram of solvent.
Important Topic:
| CBSE Class 12 NCERT Notes | |
| NCERT Class 12 Maths Notes for CBSE |
Factors Affecting Depression in Freezing Point
The following factors affect freezing point depression.
- Amount of solute
- Solute and Solvent nature
- Cryoscopic constant (Kf) of the solvent
Freezing Point and Depression of Freezing Point for Solvents
Check the table to know the freezing point and the depression freezing point for various solvents.
| Solvent | Freezing Point (f. p / K) | Freezing Point Depression |
| Water | 273.0 | 1.86 |
| Benzene | 278.6 | 5.12 |
| Chloroform | 209.6 | 4.79 |
| Acetic acid | 290.0 | 3.90 |
| Ethanol | 155.7 | 1.99 |
| Diethyl ether | 156.9 | 1.79 |
Also Check:
| NCERT Class 11 Notes | |
| Class 11 Chemistry NCERT Notes |
Uses of Freezing Point Depression
The following are some important uses of freezing point depression.
- Using the freezing point depression formula, the molar mass of a given solute can be calculated
- The degree to which a solute dissociates in a solvent can also be calculated using the freezing point depression formula
- In very cold areas where the temperatures range from 0oC to -15oC, NaCl is spread over the roads in order to lower the freezing point of water and thus prevent the formation of ice.
- Radiator fluids which are used in many automobiles are usually made up of ethylene glycol and water. This prevents the freezing of the radiator during extreme cold seasons.
The freezing point of a solution is lower than that of the pure solvent due to the presence of solute particles, and the extent of this depression is determined by the molality of the solute and the cryoscopic constant of the solvent. This property is widely used in various fields, including chemistry and food science, to determine the concentration of solutes in solutions.
FAQs on Depression in Freezing Point
Q: A 5% solution (by mass) of cane sugar in water has freezing point of 271K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
A:
5% solution means 5g of cane sugar is present in 100g of solution
Freezing point of solution = 271k
Freezing point of pure water = 273.15k
Molar mass of cane sugar(C12H22O11) = 12 × 12 + 1 × 22 + 16 × 11 = 342g
Moles of cane sugar = mass/molar mass = 5/342
⇒ n = 0.0146mol
Molality of solution = moles of solute/mass of solvent(in kg)
⇒ M = 0.0146/0.095
⇒ Molality = 0.154M
Depression in freezing point = ΔTf = 273.15-271 = 2.15k
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ Kf = 2.15/0.154
⇒ Kf = 13.96k kg mol-1
Second condition: mass of glucose = 5g
Molar mass of glucose(C6H12O6) = 12 × 6 + 1 × 12 + 16 × 6 = 180g
Moles of glucose = mass/molar mass
⇒ n = 5/180moles
⇒ n = 0.0278mol
Molality of solution = moles of solute/mass of solvent(in kg)
⇒ molality = 0.0278/0.095
⇒ M = 0.2926M
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ ΔTf = 13.96 × 0.2926
⇒ ΔTf = 4.08k
Freezing point of solution = 273.15 + 4.08 = 277.234k
Q: Two elements A and B form compounds having formula AB2 and AB4 . When dissolved in 20 g of benzene (C6H6 ), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol^–1. Calculate atomic masses of A and B
A: let the molar masses of AB2 and AB4 be x and y respectively.
Molar mass of benzene(C6H6) = 12 × 6 + 1 × 6 = 78 g/mol
Moles of benzene = mass/molar mass = 20/78
n = 0.256mol
⇒ ΔTf = 2.3 K
Kf = 5.1K kg mol-1
For AB2
Applying the formula: ΔTf = Kf × M
Where
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 2.3 = 5.1 × M1
⇒ M1 = 0.451mol/kg
For AB4
Applying the formula: ΔTf = Kf × M
ΔTf = depression in freezing point
Kf = molal depression constant
M = molality of solution
⇒ 1.3 = 5.1 × M2
⇒ M2 = 0.255mol/kg
M1 = moles of solute/mass of solvent(in kg)
M1 = 1/x / 0.02 = 1 / 0.02x = 0.451
⇒ X = 110.86g
M2 = moles of solute/mass of solvent(in kg)
M2 = 1/y / 0.02 = 1 / 0.02y = 0.255
⇒ y = 196.1g
atomic mass of A be a and that of B be b g respectively.
So, AB2 : a + 2b = 110.86
AB4: a + 4b = 196.1
⇒ a = 25.59g
⇒ b = 42.64g
Q: The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
A:
The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the pattern,
Acetic acid< trichloroacetic acid< trifluoroacetic acid.
This is because fluorine is more electronegative than chlorine. So, trifluoracetic acid is a stronger acid in comparison to trichloroacetic acid and acetic acid. And also, acetic acid is the weakest of all.
Explanation: Stronger acid produces more number of ions, therefore it has more ΔTf(depression in freezing point), hence lower freezing point. As the acidic strength increases, the acid gets more and more ionised.
Trifluoracetic acid ionizes to the largest extent. Hence, in this case, trifluoracetic acid being the strongest acid produces more number of ions(extent of ionisation and concentration of ions are more), high ΔTf(depression in freezing point)and lower freezing point and vice versa.
Q: Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. Ka = 1.4 × 10^–3 , Kf = 1.86 K kg mol^–1 .
A:
Mass of CH3CH2CHClCOOH = 10 g
Mass of water = 250g
Ka = 1.4 × 10–3,
Kf = 1.86 K kg mol–1
Molar mass of CH3CH2CHClCOOH = 12 + 3 + 12 + 2 + 12 + 1 + 35.5 + 2 + 16 + 16 + 1
= 122.5 g mol–1
Number of moles of solute = Mass of Solute / Molar Mass
→ No. of moles = 10g / 122.5 g/mol
∴ No. of moles = 8.6 X 10–2 mol
Now, Molality is given as,
M = Number of moles of solute / kg of solvent
M= 8.6 X 10–2 X 1000 g/mol / 250 g
M = 0.3264 kg/mol
CH3CH2CHClCOOH = CH3CH2CHClCOO- + H +
| Initial moles |
1 |
0 |
0 |
|
Equilibrium moles |
(1-α) |
α |
α |
Total moles at equilibrium = (1-α) + 2 α
= 1 + α
In order to find out the depression in freezing point,

values of i(vant Hoff’s factor) and α(degree of dissociation) are to be found out.
To find out degree of dissociation, α

∴ i = 1.0654
Now, to find out the depression in freezing point,

Q: 19.5 g of CH2FCOOH is dissolved in 500 g of water. The depression in the freezing point of water observed is 1.00 C. Calculate the van’t Hoff factor and dissociation constant of fluoroacetic acid.
A:
Given- w1 = 500g
W2 = 19.5g
Kf = 1.86 K kg mol-1
Molar mass of CH2FCOOH = 12 + 2 + 19 + 12 + 16 + 16 + 1
= 78 g mol-1
The depression in freezing point is calculated by,

→ (where, m is the molality)
= 1.86 X 19.5 / 78 X 1000/500
= 1.86 X 19.5 / 78 X 2
=0.93
∴ Δtf (calculated) = 0.93
To find out the vant Hoff’s factor, we use the formula,
i = observed Δtf / calculated Δtf
i = 1.0 (given) / 0.93
∴ i= 1.07
CH2FCOOH → CH2FCOO- + H +
To find out the degree of dissociation α, we use

Thus, the vant Hoff’s factor is 1.07 an the dissociation constant is 2.634x10-3
Chemistry Atoms and Molecules Exam
Student Forum
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test