
NCERT Chemistry Class 11 S-Block Elements: S block is the elements of group 1 and 2 of the periodic table. S-block elements of group 1 consist of the elements lithium, sodium, potassium, rubidium, caesium and francium. They are collectively known as the alkali metals. The elements of group 2 include beryllium, magnesium, calcium, strontium, barium and radium. These elements, with the exception of beryllium, are commonly known as the alkaline earth metals.
CBSE Class 11 Chemistry s-Block chapter comprises topics such as group 1- Alkali Metals and Group 2 – Alkaline Earth Metals, Characteristics of Compounds of Alkaline Earth Metals, Oxides and hydroxides of s-Block Elements, Hydrogen bonding and solvation, Some Important Compounds of Calcium, Anomalous Properties of Lithium and Behaviour of Beryllium and more. In addition to this, students will also understand the uses of s-block elements in industries, medical and household work.
Students are provided here the NCERT class 11 chemistry solution for s-block elements. The subject experts at Shiksha have prepared the Class 11 Chemistry S-block elements NCERT solutions, including textbook exercises and previous year questions. Students can use the NCERT solutions for Class 11 Chemistry to prepare for the boards and competitive exams such as JEE Main, NEET, MHT CET, etc. Moreover, students must practice the textbook questions and answers for s-Block Elements to know how well they have understood the topics. Additionally, it is important to solve the NCERT Solutions for Class 11 Chapter 10 The s-block Elements after reading all topics and clearing the basic doubts. Students can also find Class 11 NCERT Solutions Chapter Wise pdf link on this page.
NCERT Chemistry Class 11 Chapter 10 solutions PDF is considered the best resource when it comes to preparing for the exam. The toppers of various competitive exams have suggested focusing on Class 11 Chemistry NCERT Chapter 10 exercise solutions and previous year questions will help to obtain good marks. Below is the link for NCERT Chemistry solutions.
- NCERT Class 11 Chemistry S Block Elements Topics Covered
- The S-block Elements Solutions
NCERT Class 11 Chemistry S Block Elements Topics Covered
Students can check here the list of all the topics that are covered in s-block element chapter of NCERT Chemistry class 11.
- Group 1 Element- Alkali Metals
- Electronic Configuration
- Atomic and Ionic Radii
- Ionization Enthalpy
- Hydration Enthalpy
- Physical Properties
- Chemical Properties
- Uses
- General characteristics of compounds of Alkali elements
- Oxides and Hydroxides
- Halides
- Salts of Oxo-Acids
- Anomalous Properties of Lithium
- Points of Difference between Lithium and other Alkali Metals
- Points of Similarities between Lithium and Magnesium
- Some Important Compounds of Sodium
- Sodium Carbonate (Washing Soda)
- Sodium Hydroxide (Caustic Soda)
- Sodium Hydrogen carbonate (Baking Soda)
- Biological Importance of Sodium and Potassium
- Group 2 Elements- Alkaline Earth Metals
- Electronic Configuration
- Atomic and Ionic Radii
- Ionization Enthalpy
- Hydration Enthalpy
- Physical Properties
- Chemical Properties
- Uses
- General characteristics of compounds of Alkaline Earth Metals
- Anomalous Properties of Beryllium
- Some Important Compounds of Calcium
- Calcium Oxide (Quick Lime)
- Calcium Hydroxide (Slaked Lime)
- Calcium Carbonate
- Calcium Sulphate (Plaster of Paris)
- Biological Importance of Calcium and Magnesium
The S-block Elements Solutions
| 10.1. What are the common physical and chemical features of alkali metals? |
Common chemical features of alkali metals are: The alkali metals are highly reactive due totheir large size and low ionization enthalpy. Thereactivity of these metals increases down thegroup.
|
| 10.2. Discuss the general characteristics and gradation in properties of alkaline earth metals. |
| Answer: The general characteristics and gradation in properties of alkaline earth metals are:
|
| 10.3. Why are alkali metals not found in nature? |
| Answer: All the alkali metals have one valence electron, ns1 outside the noble gas core. The loosely held s-electron in the outermost valence shell of these elements makes them the mostelectropositive metals, i.e. they readily lose electron to give monovalent M+ ions. Hence, they are never found in free state in nature. |
| 10.4. Find out the oxidation state of sodium in Na2O2. |
| Answer: Let x be the oxidation state of Na in Na2O2 Then, 2x + 2 (-1) = 0 =>2x – 2 = 0 => x = +1. |
Commonly asked questions
10.15. Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals.
(a) Nitrates (b) Carbonates (c) Sulphates.
(a) Nitrates of both group 1 and group 2 elements are soluble in water because hydration energy is more than the lattice energy.
Nitrates of both group 1 and group 2 elements are thermally unstable but they decompose differently except LiCO3 e.g.
2NaNO3 →2NaNO2 + O2
2KNO3 →2KNO2 + O2
4LiNO3 →2MgO + 4 NO2 + O2
2Mg (NO3)2 →2MgO + 4NO2 + O2
(b) Carbonates of group 1 elements are soluble in water except Li2CO3 They are also thermally stable except Li2CO3.
Li2CO3 →Li2O + CO2
Group 2 carbonates are insoluble in water because their lattice energy is higher than hydration energy.
Thermal stability of carbonates of group 2 increases down the group because lattice energy goes on increasing due to increase in ionic character.
(c) Sulphates of group 1 are soluble in water except Li2SO4. They are thermally stable.
Solubility of sulphates of group 2 decreases down the group because Lattice energy dominates over hydration energy.
Sulphates of group 2 elements are thermally stable and increasing down the group due to increases in Lattice energy.
10.32. Which one of the alkaline earth metal carbonates is thermally most stable?
(a) MgCO3 (b) CaCO3 (c) SrCO3 (d) BaCO3(Intermediate)
(d) BaCO3
Thermal stability is directly proportional to the size of the cation i.e., larger the size of the atom, greater is its thermal stability. Ba being the biggest cation among the given compounds, has a greater thermal stability.
10.29. How would you explain the following observations?
(i) BeO is almost insoluble but BeSO4 is soluble in water,
(ii) BaO is soluble but BaSO4is insoluble in water,
(iii) LiI is more soluble than KI in ethanol.
(i) Lattice energy of BeO is comparatively higher than the hydration energy. Therefore, it is almost insoluble in water. Whereas, BeSO4 is ionic in nature and its hydration energy dominates the lattice energy.
(ii) Both BaO and BaSO4 are ionic compounds but the hydration energy of BaO is higher than the lattice energy therefore it is soluble in water.
(iii) Since the size of Li+ ion is very small in comparison to K+ ion, it polarises the electron cloud of I– ion to a great extent. Thus, Lil dissolves in ethanol more easily than the KI.
10.31. Which one of the following alkali metals gives hydrated salts?
(a) Li (b) Na (c) K (d) Cs (Intermediate)
(a) Li
Li+ is the smallest in size and thus, has the highest charge density and hence attracts the water molecules more strongly to form hydrated salts.
10.30. Which of the alkali metal is having least melting point?
(a) Na (b) K (c) Rb (d) Cs
(d) Cs
Size of Cs is the biggest thus, its melting point is the lowest, (d) is correct
10.1. What are the common physical and chemical features of alkali metals?
- Physical appearance: All the alkali metals are silvery white, soft and light metals.
- Density: Because of the large size, these elements have low density which increases down the group except for potassium which is lighter than sodium (most likely due to an unexpected increase in the atomic size.).
- The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them.
- Atomic volume:The atomic volume, atomic and ionic radii rise as the group number reduces from Li to Cs.
- Melting and boiling points:The weak crystal lattice bonding causes low melting and boiling points of all alkali metals.
- The alkali metals and their salts impart characteristiccolour to an oxidizing flame and thus can be detected bythe respective flame tests and can bedetermined by flame photometry or atomic absorption spectroscopy.
- These elements whenirradiated with light, the light energy absorbedmay be sufficient to make a can atom lose electron.This property makes caesium and potassiumuseful as electrodes in photoelectric cells.
- Ionisation power:Alkali metals have the lowest initial ionisation energy among their periods.The ionisation energy drops as one moves down the group.
- Electropositivity character:Alkali metals are highly electropositive or metallic in nature, and this feature grows in strength as the alkali metals progress from Li to Cs.
- Electrode potentials:Alkali metals are strongly electropositive and oxidise easily in water, releasing hydrogen gas.
- Reducing nature:Due to their low ionisation energies, alkali metals are good reductants. Their lowering character is Na, Cs, Rb, K, Li (in aqueous solution).
- Electronegativity:The electronegativity of alkali metals decreases as one moves down the periodic table.
- Oxidation states:The outermost valence shell of all alkali metals contains only one electron. After the penultimate shell is completed, these atoms lose one electron to the nearest inert gas. As a result, the monovalent elements have an oxidation state +1.
- Hydration of ions:All alkali metal salts (excluding lithium) are ionic and soluble in water due to a strong hydration tendency of the alkali metal cations;
- Colour of flame:All alkali metals and associated salts give a non-luminous flame a distinct colour.Because of the absorption of energy, the electrons of an alkali metal and its salt are stimulated to higher energy levels when placed in a burning flame. This is a volatile state. Thus, a specific light colour is released as the electrons return towards the ground state. As one moves from Li to Cs, the ionisation energy decreases, increasing the frequency of the light emitted.
- The photoelectric effect:Alkali metals, in particular K and Cs, have a photoelectric effect because of their low ionisation activity and work function.
- The electrical conductivity:Since the valence electrons of all alkali metals may freely travel through the crystal structure, they are excellent heat and electric conductors. Electrical conductivity increases as one moves down the group.
Common chemical features of alkali metals are:
The alkali metals are highly reactive due totheir large size and low ionization enthalpy. Thereactivity of these metals increases down thegroup.
- Reactivity towards air: The alkali metalstarnish in dry air due to the formation oftheir oxides which in turn react withmoisture to form hydroxides. They burnvigorously in oxygen forming oxides.Because oftheir high reactivity towards air and water, alkali metals are normally kept in kerosene oil.
- Reactivity towards water: The alkalimetals react with water to form hydroxideand dihydrogen. Reaction of lithium with water is less vigorous thanthat of sodium due to itssmall size and very high hydration energy.Other metals of the group react explosivelywith water.They also react with proton donors suchas alcohol, gaseous ammonia and alkynes.
- Reactivity towards dihydrogen: Thealkali metals react with dihydrogen atabout 673K to formhydrides. All the alkali metal hydrides areionic solids with high melting points.
- Reactivity towards halogens: The alkalimetals readily react vigorously withhalogens to form ionic halides, butlithium halides are somewhatcovalent due to highpolarisation capability of lithium ion.
- Reducing nature: The alkali metals arestrong reducing agents, lithium being themost and sodium the least powerful. With the small size of its ion, lithium hasthe the highest hydration enthalpy whichaccounts for its high negative E0 value andits high reducing power.
- Solutions in liquid ammonia: The alkalimetals dissolve in liquid ammonia givingdeep blue solutions which are conductingin nature.
10.25. What happens when
(i) Sodium metal is dropped in water?
(ii) Sodium metal is heated in free supply of air?
(iii) Sodium peroxide dissolves in water?
(i) 2Na + 2H2O → 2NaOH + H2
(ii) 2Na + O2 → Na2O2
(iii) Na2O2 + 2H20 → 2NaOH + H2O2
10.2. Discuss the general characteristics and gradation in properties of alkaline earth metals.
The general characteristics and gradation in properties of alkaline earth metals are:
- Atomic size goes on increasing down the group.
- Ionisation energy goes on decreasing down the group.
- They are harder than alkali metals.
- They are less electropositive than alkali metals.
Electropositive character increases on going down the group.
10.3. Why are alkali metals not found in nature?
All the alkali metals have one valence electron, ns1 outside the noble gas core. The loosely held s-electron in the outermost valence shell of these elements makes them the mostelectropositive metals, i.e. they readily lose electron to give monovalent M+ ions. Hence, they are never found in free state in nature.
10.4. Find out the oxidation state of sodium in Na2O2.
Let x be the oxidation state of Na in Na2O2
Then, 2x + 2 (-1) = 0
=>2x – 2 = 0
=> x = +1.
10.14. Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Li2CO3 is a covalent compound whereas Na2CO3 is an ionic compound. Therefore, lattice energy of Na2CO3 is higher than that of Li2CO3. Thus, LiCO3 is decomposed at a lower temperature as compared to Na2CO3.
10.18. Describe two important uses of each of the following:
(i) caustic soda (ii) sodium carbonate (iii) quicklime.
(i) Caustic soda
It is used in (a) the manufacture of soap, paper, artificial silk and a number of chemicals, (b) in petroleum refining, (c) in the purification of bauxite, (d) in the textile industries for mercerising cotton fabrics, (e) for the preparation of pure fats and oils, and (f) as a laboratory reagent.
(ii) Sodium carbonate
(a) It is used in water softening, launderingand cleaning.
(b) It is used in the manufacture of glass, soap, borax and caustic soda.
(c) It is used in paper, paints and textileindustries.
(d) It is an important laboratory reagent bothin qualitative and quantitative analysis
(iii) Quick lime
(a) It is an important primary material formanufacturing cement and is the cheapest
form of alkali.
(b) It is used in the manufacture of sodiumcarbonate from caustic soda.
(c) It is employed in the purification of sugarand in the manufacture of dye stuffs.
10.21. Describe the importance of the following:
(i) Limestone (ii) Cement (iii) Plaster of paris.
Limestone:
- A raw material for cement.
- It is used as a building material in the form ofmarble and in the manufacture of quick lime.
- used in the manufactureof high quality paper. It is also used as anantacid, mildabrasive in tooth paste, aconstituent of chewing gum, and a filler incosmetics.
Cement:
- It is used in concrete and reinforcedconcrete, in plastering and in the construction
- of bridges, dams and buildings
Plaster of Paris:
- It is used in the building industry as well as plasters.
- It is usedfor immobilising the affected part of organ wherethere is a bone fracture or sprain.
- It is alsoemployed in dentistry, in ornamental work andfor making casts of statues and busts.
10.24. Explain the significance of sodium, potassium, magnesium and calcium in biological fluids.
Sodium:
- Na+ions participate in the transmission of nerve signals, in regulating the flow of water across cell membranes.
- In the transport of sugars and amino acids into cell.
Potassium:
- K+ions activate many enzymes.
- Participate in the oxidation of glucose to produce ATP.
Magnesium:
- All enzymes that utilise ATP in phosphate transfer require magnesium as a cofactor.
- Mg is the central metal ion present in chlorophyll pigment in plants.
Calcium:
- Ca2+ ions are present in bones.
- plays important roles in neuromuscular function.
10.2. Assertion: The ionization enthalpies of the alkali metalsare considerably high and increase down thegroup from Li to Cs.
Reason: The effectof increasing size outweighs the increasingnuclear charge, and the outermost electron isvery well screened from the nuclear charge.
(d) The ionization enthalpies of the alkali metals are considerably low and decrease down the group from Li to Cs. This is because the effect of increasing size outweighs the increasing nuclear charge, and the outermost electron is very well screened from the nuclear charge.
Question Answers:
10.1. Explain the Diagonal Relationship betweenBeryllium and Aluminium.
The ionic radius of Be2+ is estimated to be 31 pm; the charge/radius ratio is nearly the same as that of the Al3+ ion. Hence beryllium resembles aluminium in some ways. Some ofthe similarities are:
(i) Like aluminium, beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium hydroxide dissolves in excess of alkali to give a beryllate ion, [Be (OH)4]2– just as aluminium hydroxide gives aluminate ion, [Al (OH)4]–.
(iii) The chlorides of both beryllium and aluminium have Cl– bridged chloride structure in vapour phase. Both the chlorides are soluble in organic solventsand are strong Lewis acids. They are usedas Friedel Craft catalysts.
(iv) Beryllium and aluminium ions have strong tendency to form complexes, BeF42–, AlF6
10.2. What are group I elements and why are they known as the alkali metals?
Lithium, sodium, potassium, rubidium, caesium and Francium are group I elements and collectively known as the alkali metals.
They are called as the alkali metals because they form hydroxides on reaction with water which are strongly alkaline in nature.
10.10. The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain.
All the compounds are crystalline solids and their solubility in water is guided by both lattice enthalpy and hydration enthalpy. In case of sodium and potassium compounds, the magnitude of lattice enthalpy is quite small as compared of sodium and potassium that are mentioned, readily dissolve in water. However, in case of corresponding magnesium and calcium compounds, the cations have smaller sizes and more magnitude of positive charge. This means that their lattice enthalpies are more as compared to the compounds of sodium and potassium. Therefore, the hydroxides and carbonates of these metals are only sparingly soluble in water.
10.5. Explain why sodium is less reactive than potassium.
It is because ionization enthalpy? Hi of potassium = 419 kJ mol-1. Ionization enthalpy of sodium = 496 KJ mol-1. Since Ionization enthalpy of potassium is less than that of sodium, potassium is more reactive than sodium.
10.6. Compare the alkali metals and alkaline earth metals with respect to (i) ionisation enthalpy (ii) basicity of oxides and (iii) solubility of hydroxides.
(i) Ionization enthalpy. Because of high nuclear charge the ionization enthalpy of alkaline earth metals are higher than those of the corresponding alkali metals.
(ii) Basicity of oxides. Basicity of oxides of alkali metals are higher than that of alkaline earth metals.
(iii) Solubility of hydroxides of alkali metals is higher than that of alkaline earth metals. Alkali metals due to lower ionization enthalpy are more electropositive than the corresponding group 2 elements.
10.7. In what ways lithium shows similarities to magnesium in its chemical behaviour?
Both lithium and magnesium are harder and lighter than other elements in the respective groups.
Lithium and magnesium react slowly with water. Their oxides and hydroxides are much less soluble and their hydroxides decompose on heating. Both form a nitride, Li3N and Mg3N2, by direct combination with nitrogen.
The oxides, Li2O and MgO do not combine with excess oxygen to give any superoxide. (iv) The carbonates of lithium and magnesium decompose easily on heating to form the oxides and CO2. Solid hydrogen carbonates are not formed by lithium and magnesium.
Both LiCl and MgCl2are soluble in ethanol.
Both LiCl and MgCl2are deliquescent and crystallise from aqueous solution as hydrates, LiCl·2H2O and MgCl26H2
10.8. Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods?
Alkali and alkaline earth metals are reducing agents. That is why these metals are not obtained by chemical reduction methods.
10.9. Why are potassium and caesium, rather than lithium used in photoelectric cells?
Potassium and caesium have much lower ionization enthalpy than that of lithium. As a result, these metals easily emit electrons on exposure to light. Due to this, K and Cs are used in photoelectric cells rather than lithium.
10.10. When an alkali metal dissolves in liquid ammonia, the solution can acquire different colours. Explain the reasons for this type of colour change.
Different concentrations of alkali metals in liquid ammonia gives different colours. The dilute solutions impart blue colour due to presence of ammoniated electrons whereas the concentrated solutions have copper bronze colour as ammoniated metal ions are bound by free electrons.
10.11. Beryllium and magnesium do not give colour to flame whereas other alkaline earth metals do so. Why?
Due to small size, the ionization enthalpies of Be and Mg are much higher than those of other alkaline earth metals. Therefore, a large amount of energy is needed to excite their valence electron, and that's why they do not impart colour to the flame.
10.12. Discuss the various reactions that occur in the Solvay process.
Solvay process is also known as ammonia soda process, wherein carbon dioxide is passed through a brine solution (containing about 28 % NaCl) which is saturated with ammonia to form sodium carbonate.
2NH3 + H2O + CO2 → (NH4)2CO3
(NH4)2CO3 + H2O + CO2 → 2NH4HCO3
NH4HCO3 + NaCl → NaHCO3↓ + NH4Cl
The precipitate of sodium bicarbonate is filtered, dried and ignited to form sodium carbonate.
2NaHCO3? →? Na2?CO3? + CO2 ?+ H2?O
The carbon dioxide required for the reaction can be obtained by heating limestone (calcium carbonate) to 1300 K in a lime klin. Lime dissolves in water to form calcium hydroxide which is then transferred to the ammonia recovery tower.
CaCO3?→? ?CaO+CO2?
CaO+H2?O→Ca(OH)2?
Ammonia required for the process can be prepared by heating ammonium chloride with calcium hydroxide.
2NH4?Cl+Ca(OH)2→ ?2NH3?+CaCl2?+H2?O
Hence, the only by product of the reaction is calcium chloride.
10.13. Potassium carbonate cannot be prepared by Solvay process. Why?
Potassium carbonate cannot be prepared by Solvay process because potassium hydrogencarbonate is too soluble to be precipitated by the addition of ammonium hydrogencarbonate to a saturated solution of potassium chloride.
10.17. What happens when (i) magnesium is burnt in air, (ii) quick lime is heated with silica (iii) chlorine reacts with slaked lime (iv) calcium nitrate is heated?
(i) Magnesium is burnt in air to form magnesium oxide and magnesium nitride.
2Mg + O2 → 2MgO
3Mg + N2 → Mg3N2.
(ii) Quick lime is heated with silica above 1273 K to obtain calcium silicate
CaO+SiO2 → CaSiO3.
(iii) Chlorine reacts with slaked lime to form calcium hypochlorite- a constituent of bleaching powder.
2Ca (OH)2 + 2Cl2 → CaCl2 + Ca (OCl)2 + 2H2O.
(iv) Calcium nitrate is heated to obtain CaO, NO2 and O2.
2Ca (NO3)2→2CaO+4NO2+O2.
10.19. Draw the structure of (i) BeCl2 (vapour), (ii) BeCl2 (solid).
BeCl2 (vapour)
In the vapour state, it exists as a chloro-bridged dimer.
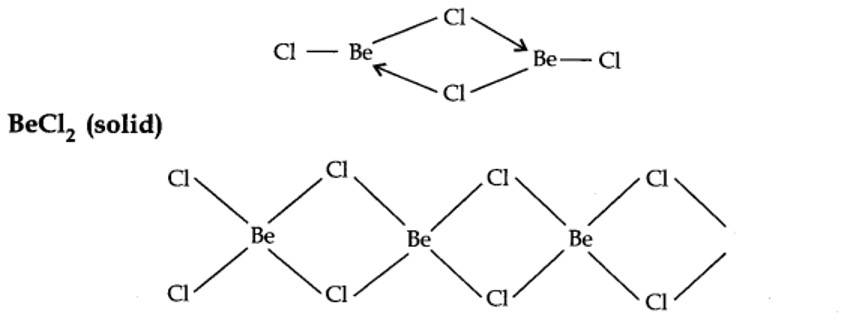
10.20. The hydroxides and carbonates of sodium and potassium are easily soluble in water while the corresponding salts of magnesium and calcium are sparingly soluble in water. Explain.
Since group 1 hydroxides and carbonates due to large size contain higher hydration energy than the lattice energy so, they are easily soluble in water. Whereas, in magnesium and calcium due to small size their lattice energy dominates over hydration energy they are sparingly soluble in water.
10.22. Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Li+ can polarize water molecules easily than the other alkali metal ions because of its small size.
10.23. Why is LiF almost insoluble in water whereas LiCl soluble not only in water but also in acetone?
It is due to high lattice energy of LiF as compared to LiCl.
LiCl is soluble in water because its hydration energy is higher than its lattice energy.
10.26. Comment on each of the following observations:
(a) The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+
(b) Lithium is the only alkali metal to form a nitride directly.
(c) E? for M2+ (aq) + 2e– → M(s) (where M = Ca, Sr, or Ba) is nearly constant.
(a) Smaller the size of the ion, more highly it is hydrated and hence greater is the mass of the hydrated ion and thus the ionic mobility become lesser. The extent of hydration decreases in the order.
Li+ < Na+
Thus the mobility of Cs+ will be the highest
(b) Due to its smaller size lithium can form nitride directly as an exceptional behaviour unlike other alkali metals.
(c) It is because reduction potential depends upon sublimation energy, ionisation energy and hydration energy. Their resultant is almost constant for these ions.
10.27. State as to why
(a) a solution of Na2CO3 is alkaline?
(b) alkali metals are prepared by electrolysis of their fused chlorides?
(c) sodium is found to be more useful than potassium?
(a) Na2CO3 is a salt of a weak acid, carbonic acid (H2CO3) and a strong base NaOH. Thus, it undergoes hydrolysis to produce strong base NaOH and its aqueous solution isalkaline in nature.
Na2CO3 (s) + H2O (l) → 2NaOH
(b) Because the discharge potential of alkali metals is much higher than that of hydrogen, therefore when the aqueous solution of any alkali metal chloride is subjected to electrolysis, H2, instead of the alkali metal, is produced at the cathode. Therefore, alkali metals are prepared by electrolysis of their fused chlorides.
(c) Since potassium is move reactive than sodium and it is found in nature to a less extent than Na, sodium is found to be more useful.
10.28.Write balanced equations for reactions between
(a) Na2O2 and water
(b) KO2 and water
(c) Na2O and CO2
(a) Na2O2 + 2H2O → 2NaOH + H2O2
(b) 2KO2 + 2H2O → 2KOH + O2+ H2O2
(c) Na2O+ CO2 → Na2CO3
10.4. Assertion: Alkali metals are always univalent.
Reason: Alkali metals are soft.
(b) Both the statements are correct but not the reason for the assertion.
10.5. Assertion: Electrode potential is a measure of the tendency of an element to lose electrons in the aqueous solution. So, the reducing property can be correlated in terms ofelectrode potentials (E°) of alkali metals.
Reason: More positive is the electrode potential, higher is the tendency of the element to loose electrons and hence, stronger is the reducing agent.
(c) Reason is wrong a statement. More negative is the electrode potential, higher is the tendency of the element to lose electrons and hence, stronger is the reducing agent.
10.5. Arrange the following in the increasing order of solubility in water.
(a) MgCl2 < CaCl2< SrCl2< BaCl2
(b) BaCl2 < SrCl2 < CaCl2
(c) SrCl2 < MgCl2 < CaCl2 < BaCl2
(d) MgCl2 < CaCl2< BaCl2< SrCl2
(b) BaCl2 < SrCl2 < CaCl2
10.3. Why BeSO4 is soluble in water while BaSO4 is not?
Hydration energy decreases down the group from Be to Ba and lattice energy remains almost constant.
10.4. Why Lithium salts are hydrated?
Li+ has maximum degree of hydration due to its small size and for this reason lithium salts are mostly hydrated.
10.5. Why are s-block elements highly electropositive?
The loosely held s-electron in the outermost valence shell of these elements makes them the most electropositive metals which readily give ions, M+ or M2+.
10.6. Why Beryllium carbonate is kept in the atmosphere of carbon dioxide?
Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
10.7. Explain the following:
(a) Why Cs is considered as the most electropositive element?
(b) Lithium cannot be used in making photoelectric cells.
(c) Lithium does not form alums.
(a) Due to its the lowest ionization energy, Cs is considered as the most electropositive element.
(b) Lithium cannot be used in making photoelectric cells because out of all the alkali metals it has the highest ionization energy and thus cannot emit electrons when exposed to light.
(c) Due to small size, lithium does not form alums
10.8. State as to why
(a) Alkali metals show only +1 oxidation state. (b) Na and K impart colour to the flame but Mg does not.(c) Lithium on being heated in air mainly forms the monoxide and not the peroxide.(d) Li is the best reducing agent in aqueous solution.
(a) Alkali metals have low ionization enthalpies.
They have a strong tendency to lose 1 electron to form unipositive ions. Thus they show an oxidation state of +1 and are strongly electropositive.
(b) Valence electrons of alkali metals like Na and K easily absorb energy from the
flame and are excited to higher energy levels. When these electrons return to the ground state, the energy is emitted in the form of light.
Magnesium atom has small size so electrons are strongly bound to the nucleus. [ Thus they need large amount of energy for excitation of electrons to higher
energy levels which is not possible in Bunsen flame.
(c)Due to the small size of Li+ it has a strong positive field which attracts the negative charge so strongly that it does not permit the oxide ion, 02- to combine with another oxygen atom to form peroxide ion.
(d)Since, among alkali metals, lithium has the most negative electrode potential (E° = -3.04 V) so, it is the strongest reducing agent in the aqueous solution.
10.9. Potassium carbonate cannot be prepared by Solvay process. Why?
This is due to the reason that potassium bicarbonate (KHCO3) formed as an intermediate (when CO2 gas is passed through ammoniated solution of potassium chloride) is highly soluble in water and cannot be separated by filtration.
10.1.Assertion: The elements of Group 2 are commonly known asthe alkaline earth metals.
Reason: Theiroxides and hydroxides are alkaline in nature and thesemetal oxides are found in the earth’s crust.
Kindly go through the solution
(a)
10.3. Assertion: The alkalimetals and their salts impart characteristiccolour to an oxidizing flame.
Reason: This heat from the flame excites the outermost orbitalelectron to a higher energy level. When the excitedelectron comes back to the ground state, thereis emission of radiation in the visible region ofthe spectrum.
Kindly go through the solution
(a)
10.1. Lithium shows a diagonal relationship with
(a) Sodium (b) Silicon (c) Nitrogen (d) Magnesium
Kindly go through the solution
(d)
10.2. In the Solvay process
(a) An ammoniacal brine solution is carbonated with CO2, forming NaHCO3 which on decomposition at 150°C produces Na2CO3
(b) A sodium amalgam reacts with water to produce NaOH which gives Na2CO3 on reacting with CO2
(c) A brine solution is made to react with BaCO3 to produce Na2CO3
(d) All of the above
Kindly go through the solution
(a)
10.3. Which of the following is not a peroxide?
(a) KO2 (b) CrO5 (c) Na2O2 (d) BaO2
Kindly go through the solution
(a)
10.4. Salt of which alkaline earth metals do not impart colour to a non-luminous flame.
(a) Ca (b) Mg (c) Be (d) Ba
(c) Beryllium does not impart colour to a non-luminous flame.
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
