
NCERT Solutions for Class 11 Chemistry Chapter 7 – Redox Reactions are prepared by Shiksha's experts. It offers clear, step-by-step explanations to help Class 11 students get a grasp of the complex redox processes such as oxidation, reduction, electron transfer, and balancing redox equations.
Redox Reaction Class 11 NCERT Solutions are aligned with the latest CBSE curriculum. It provides ideal study material for Class 11 exam preparation and for other competitive exam preparation. The students will understand the key topics like oxidation numbers, redox titrations, and electrochemical applications.
Get the NCERT notes for Class 11 Chemistry at - Class 11 Chemistry NCERT Notes. It offers Key topics, solved examples, and chapter-wise PDF.
- Class 11th Chemistry Redox Reactions: Key Topics, Weightage
- Class 11 Redox Reaction NCERT Solution PDF: Download PDF for Free
- Important Formulas of Chemistry Chapter 7 Redox Reactions
- Redox Reaction Chapter Subtopics
- Redox Reaction Solutions
- Redox Reaction Additional Questions
- Redox Reaction - FAQs
Class 11th Chemistry Redox Reactions: Key Topics, Weightage
Class 11th Chemistry Redox Reactions is an important chapter for CBSE Board exams and for other entrance exams. Find below the topics covered in Chapter 7:
| Exercise | Topics Covered |
|---|---|
| 7.1 | Classical Idea of Redox Reactions – Oxidation and Reduction Reactions |
| 7.2 | Redox Reactions in terms of Electron Transfer Reactions |
| 7.3 | Oxidation Number |
| 7.4 | Redox Reactions and Electrode Processes |
Class 11 Chapter 7 Chemistry Redox Reactions Weightage in NEET, JEE Main Exam
| Exam | Number of Questions | Weightage |
|---|---|---|
| NEET | 1 question | 1.90% |
| JEE Main | 1-2 questions | 3.3% |
Related Links
| NCERT Class 11 Notes | NCERT Notes for Class 11 & 12 |
| Class 11th NCERT Solutions Chemistry | NCERT Solutions Class 11 and 12 |
Class 11 Redox Reaction NCERT Solution PDF: Download PDF for Free
Students can download the NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions PDF from below to get access to the well-structured solutions. It will not only help students to solve their homework and assignments on time, but also is a great tool for quick revision for CBSE Board exam and other competitive exams NEET, and JEE Main exam.
Class 11 Chemistry Redox Reaction NCERT Solution PDF: Free PDF Download
Important Formulas of Chemistry Chapter 7 Redox Reactions
Important Reactions of Redox Reactions for CBSE and Competitive Exams
-
Reaction of Hydrogen Peroxide (H₂O₂)
- As an oxidizing agent:
- As a reducing agent:
- As an oxidizing agent:
-
Disproportionation Reaction (Self-Oxidation & Reduction)
-
Reaction of Potassium Permanganate (KMnO₄)
- With Fe²⁺ in an acidic medium:
- With Fe²⁺ in an acidic medium:
-
The reaction of Potassium Dichromate (K₂Cr₂O₇)
- With Sn²⁺ in an acidic medium:
- With Sn²⁺ in an acidic medium:
Redox Reaction Chapter Subtopics
Given here are the subtopics that students will be studying in the Chemistry class 11 NCERT solution for Redox Reactions.
- Classical Idea of Redox Reactions – Oxidation and Reduction Reactions
- Redox Reactions in Terms of Electron Transfer Reactions
- Competitive Electron Transfer Reactions
- Oxidation Number
- Types of Redox Reactions
- Combination Reactions
- Decomposition Reactions
- Displacement Reactions
- Disproportionation Reactions
- Balancing of Redox Reactions
- Redox Reactions as the Basis for Titrations
- Limitations of the Concept of Oxidation Number
- Redox Reactions and Electrode Processes.
Redox Reaction Solutions
Students can find questions and answers on the Redox Reaction below. These questions are based on the Class 11 Chemistry Redox Reaction exercise.
| 8.1. Assign oxidation number to the underlined elements in each of the following species:
|
| Answer: Let x be the oxidation number to the underlined elements in the given species: (a) NaH2PO4 (+1) + 2(+1) + x + 4 (-2) = 0 x + 3 – 8 = 0 x = +5
(b) NaHSO4 (+1) + (+1) + x + 4(-2) = 0 x – 6 = 0 x = +6 (c) H4P2O7 4 (+1) + 2x + 7 (-2) = 0 2x -10 =0 x = +5
(d) K2MnO4 2 (+1) + x + 4 (-2) = 0 x – 6 = 0 x = +6
(e) CaO2 2 + 2x = 0 x = -1
(f) NaBH4 1 + x + 4 (-1) = 0 (Since H is present as hydride ion.) x = +3
(g) H2S2O7 2 (+1) + 2x + 7 (-2) = 0 x = +6
(h) KAl(SO4)2.12H2O +1 + 3 + 2x + 8 (-2) + 12 (2 x 1 - 2) = 0 x = +6 |
| 8.2. What is the oxidation number of the underlined elements in each of the following and how do you rationalise your results? |
| Answer: (a) In Kl3, since the oxidation number of K is +1, therefore, the average oxidation number of iodine = -1/3. But the oxidation number cannot be fractional. Therefore, we must consider its structure, K+[I —I <— I]–. Here, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine atoms forming the I2 molecule is zero, while that of iodine forming the coordinate bond is -1. Thus, the oxidation number of the three I atoms, atoms in Kl3 is 0, 0 and -1, respectively.
(b) By conventional method O.N. of S in H2S4O6is calculated as: 2 (+1) +4x + 6) (-2) = 0 Or x = +2.5 But all the four S atoms cannot be in the same oxidation state. So by chemical bonding method: The O.N. of each of the S-atoms linked with each other in the middle is zero while that of each of the remaining two S-atoms is +5. (c) By conventional method O.N. of Fe in Fe3O4 is calculated as: 3x + 4 (-2) = 0 or x = 8/3 By stoichiometry O.N. of Fe in Fe3O4 is +2 and +3. (d) By conventional method, O.N. of C in CH3CH2OH is calculated as: 2x + 6 (+1) +1 (-2) = 0 or x = -2 (e) By conventional method, O.N. of C in CH3COOH is calculated as: 2x + 4 - 4 = 0 or x = 0 By chemical bonding method, C2 is attached to three H-atoms (less electronegative than carbon) and one -COOH group (more electronegative than carbon).
Therefore, O.N. of C2 = 3 (+1) + x + 1 (-1) = 0 or x = -2 But C1 is attached to one oxygen atom by a double bond, one OH (-1) and one CH3 (+1) group, therefore, O.N. of C1 = +1 + x+ 1 (-2) + 1 (-1) = 0 or x = +2 |
| 8.3. Justify that the following reactions are redox reactions: |
| Answer:(a) Here, O is removed from CuO, therefore, it is reduced to Cu, while O is added to H2 to form H2O, therefore, it is oxidised. Further, O.N. of Cu decreases from +2 in CuO to 0 in Cu but that of H increases from 0 in H2 to +1 in H20. Therefore, CuO is reduced to Cu but H2 is oxidised to H2O. Thus, this is a redox reaction. (d) Here, each K atom as lost one electron to form K+ while F2 has gained two electrons to form two F– ions. Therefore, K is oxidised while F2 is reduced. Thus, it is a redox reaction.
(e) Here, the oxidation number of N increases from –3 in NH3 to +2 in NO. On the other hand, the oxidation number of O2 decreases from 0 in O2 to –2 in NO and H2O i.e., O2 is reduced. Hence, the given reaction is a redox reaction. |
| 8.4. Fluorine reacts with ice and results in the change: H2O(s) + F2 (g) ——> HF (g) + HOF (g) Justify that this reaction is a redox reaction. |
| Answer: Writing the O.N. of each atom above its symbol, we have, here, the O.N. of F decreases from 0 in F2 to -1 in HF and increases from 0 in F2 to +1 in HOF. Therefore, F2 is both reduced as well as oxidised. Thus, it is a redox reaction and more specifically, it is a disproportionation reaction. |
Commonly asked questions
8.26. Using the standard electrode potentials given in Table 8.1, predict if the reaction between the following is feasible:
(a) Fe3+(aq) and I-(aq) (b) Ag+ (aq) and Cu(s)
(c) Fe3+(aq) and Cu(s) (d) Ag(s) and Fe3+(aq)
(e) Br2 (aq) and Fe3+ (aq). (Advance)
(i) When emf is positive, the reaction is feasible.
Eo? cell = Eo? Fe? −Eo? I2? =0.77−0.54=0.23V
Reaction is feasible.
(ii) Eo? cell? =Eo? Ag? −Eo? Cu? =0.80−0.34=0.46V
Reaction is feasible.
(iii) Eo? cell? =Eo? Fe? −Eo? Cu? =0.77−0.34 =0.43 V
Reaction is feasible.
(iv) Eo? cell? =Eo? Fe? −Eo? Ag? =0.77−0.80=−0.03 V
Reaction is not feasible.
(v) Eo? cell? =Eo? Br2? ? −EFe? =1.09−0.77=0.32 V
Reaction is feasible.
8.12. How do you account for the following observations?
(a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
(b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless pungent smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
(a) Toluene can be oxidised to benzoic acid in acidic, basic and neutral media according to the following redox equations:
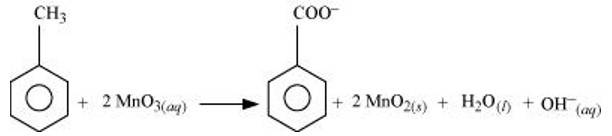
In the laboratory, benzoic acid is usually prepared by alcoholic KMnO4 oxidation of toluene. However, in industry alcoholic KMnO4 is preferred over acidic or alkaline KMnO4 because of the following reasons:
(i) The cost of adding an acid or the base is avoided because in the neutral medium, the base (OH- ions) are produced in the reaction itself.
(ii) Since reactions occur faster in homogeneous medium than in heterogeneous medium, therefore, alcohol helps in mixing the two reactants, i.e., KMnO4 (due to its polar nature) and toluene (because of its being an organic compound).
(b) KBr + H2? SO4? → KHSO4? + HBr
HBr is a strong reducing agent which further reduces H2? SO4? as follows
2HBr + H2? SO4? → Br2? + SO2? + H2? O
But in case of mixture containing Chloride the reaction ends up on forming HCl as HCl is a weak reducing agent which can't further reducethe sulphuric acid further.
KCl+H2? SO4? →KHSO4? +HCl? (colourless pungent smell gas)
8.19. Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent.
(a) P4(s) + OH–(aq) → PH3(g) + H2PO2–(aq)
(b) N2H4(l) + ClO–(aq) → NO(g) + Cl-(aq)
(c) Cl2O7(g) + H2O2(aq) → ClO2–(aq) + O2(g) + H+
(a) Oxidation number method:
The oxidation number of P decreases from 0 to -3 and increases from 0 to +2. Hence, P4? is oxidizing as well as reducing agent.
During reduction, the total decrease in the oxidation number for 4 P atoms is 12.
During oxidation, total increase in the oxidation number for 4 P atoms is 4.
The increase in the oxidation number is balanced with decrease in the oxidation number by multiplying H2? PO2−? with 3.
P4? (s) + OH− (aq) → PH3? (g) + 3H2? PO2−? (aq)
To balance O atoms, multiply OH− ions by 6.
P4? (s) + 6OH− (aq) → PH3? (g) + 3H2? PO2−? (aq)
To balance H atoms, 3 water molecules are added to L.H.S and 3 hydroxide ions on R.H.S.
P4? (s) + 6OH− (aq) + 3H2? O (l) → PH3? (g) + 3H2? PO2−? (aq) + 3OH− (aq)
Subtract 3 hydroxide ions from both sides.
P4? (s) + 3OH− (aq) + 3H2? O (l) → PH3? (g) + 3H2? PO2−? (aq)
Ion electron method:
The oxidation half reaction is
P4? (s) → H2? PO2−? (aq).
The P atom is balanced.
P4? (s) → 4H2? PO2−? (aq)
The oxidation number is balanced by adding 4 electrons on RHS.
P4? (s) → 4H2? PO2−? (aq) + 4e−
The charge is balanced by adding 8 hydroxide ions on LHS.
P4? (s)+8OH− (aq)→4H2? PO2−? (aq)
The O and H atoms are balanced.
The reduction half reaction is P4? (s)→PH3? (g).
The oxidation number is balanced by adding 12 electrons on LHS.
P4? (s)+12e−→PH3? (g)
The charge is balanced by adding 12 hydroxide ions on RHS.
P4? (s)+12e−→PH3? (g)+12OH−
The oxidation half reaction is multiplied by 3 and the reduction half reaction is multiplied by 2.
The half reactions are then added to obtain balanced chemical equation.
P4? (s)+3OH− (aq)+3H2? O (l)→PH3? (g)+3H2? PO2−? (aq)
(b) The oxidation number of N increases from – 2 in N2H4 to + 2 in NO and the oxidation number of Cl decreases from + 5 in CIO−3 to – 1 in Cl–. Hence, in this reaction, N2H4 is the reducing agent and CIO−3 is the oxidizing agent.
Ion - electron method:
The oxidation half equation is:
−2 +2
N2H4 (l)? NO (g)
The N atoms are balanced as:
N2H4 (l)?2NO (g)+8e−
The charge is balanced by adding 8 OH– ions as:
N2H4 (I)+8OH− (aq)?2NO (g)+8e−
The O atoms are balanced by adding 6H2O as:
N2H4 (I)+8OH− (aq)?2NO (g)+6H2O (I)+8e−. (i)
The reduction half equation is:
+5
8.18. Balance the following redox reactions by ion-electron method.
(a) MnO4–(aq) +I–(aq) → MnO2 (s) + I2 (s) (in basic medium)
(b) MnO4–(aq) + SO2(g) → Mn2+(aq) +H2SO4–(in acidic solution)
(c) H2O2 (aq) + Fe2+(aq) → Fe3+(aq) + H2O(l) (in acidic solution)
(d) Cr2O72- (aq) + SO2 (g) → Cr3+ (aq) + SO42-(aq) (in acidic solution)
(a) The balanced half reaction equations are:
Oxidation half equation:
I− (aq) → I2 (s) - (i)
Reduction half reaction equation:
MnO4− (aq) → MnO2 (aq) - (ii)
Balance I atoms and charges in the oxidation half reaction.
2I− (aq) → I2 (s) + 2e−
In the reduction half reaction, the oxidation number of Mn changes from +7 to +4. Hence, add 3 electrons to reactant side of the reaction.
MnO4− (aq) + 3e−→ MnO2 (aq)
Balance charge in the reduction half reaction by adding 4 hydroxide ions to product side.
MnO4− (aq) + 3e− → MnO2 (aq)+4OH−
To balance O atoms, add 2 water molecules to reactant side.
MnO4− (aq)+ 3e− +2H2O→MnO2 (aq) + 4OH−
To equalize the number of electrons, multiply the oxidation half reaction by 3 and multiply the reduction half reaction by 2.
6I− (aq) → 3I2 (s)+6e−
2MnO4− (aq) + 6e−+4H2O → 2MnO2 (aq)+8OH−
Add two half-cell reactions to obtain the balanced equation.
2MnO4−? (aq) + 6I− (aq) + 4H2? O2? (l) → 2MnO2? (s) + 3I2? (s) + 8OH−
(b) The balanced half reaction equations are:
Oxidation half equation:
SO2 (g) + 2H2O (l) → HS04– (aq) + 3H+ (aq) +2e– … (i)
Reduction half equation:
MnO4– (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (l) ………. (ii)
Multiply Eq. (i) by 3 and Eq. (ii) by 2 and add, we have,
2MnO4– (aq) + 5S02 (g) + 2H20 (l) + H+ (aq) → 2Mn2+ (aq) + 5HSO4– (aq)
(c) Oxidation half equation: Fe2+ (aq) → Fe3+ (aq) + e– … (i)
Reduction half equation: H2O2 (aq) + 2H+ (aq) + 2e– → 2H2O (l) … (ii)
Multiply Eq. (i) by 2 and add it to Eq. (ii), we have,
H2O2 (aq) +2Fe2+ (aq) +2H+ (aq) → 2Fe3+ (aq) + 2H2O (l)
(d)Oxidation half equation:
SO2 (g) + 2H2O (l) → SO42- (aq) + 4H+ (aq) + 2 e– … (i)
Reduction half equation:
Cr2O72– (aq) + 14H+ (aq) + 6e– → 2Cr3+ (aq) + 7H2O (l) … (ii)
Multiply Eq. (i) by 3 and add it to Eq. (ii), we have,
Cr2O72– (aq) + 3SO2 (q) + 2H+ (aq) → 2Cr3+ (aq) + 3SO42- (aq) + H2O (l)
8.10. The compound AgF2 is unstable compound. However, if formed, the compound acts as a very strong oxidising agent. Why?
In AgF2, oxidation state of Ag is +2 which is very unstable. Therefore, it quickly accepts an electron to form the more stable +1 oxidation state.
Ag2+ + e– →Ag+
Therefore, AgF2, if formed, will act as a strong oxidising agent.
8.29. Given the standard electrode potentials,
K+/K = -2.93 V, Ag+/Ag = 0.80 V, Hg2+/Hg = 0.79 V, Mg2+/Mg = -2.37 V,
Cr3+/Cr = -0.74 V. Arrange these metals in increasing order of their reducing power.
Lower the electrode potential, better is the reducing agent. Since the electrode potentials increase in the order: K+/K (-2.93 V), Mg2+/Mg (-2.37 V), Cr3+/Cr (-0.74 V), Hg2+/Hg (0.79 V), Ag+/Ag (0.80 V), therefore, reducing power of metals decreases in the same order, i.e., K, Mg, Cr, Hg, Ag.
8.27. Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of silver nitrate with platinum electrodes.
(iii) A dilute solution of H2SO4with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
(i) An aqueous solution of AgNO3? with silver electrodes.
At cathode: Silver ions have lower discharge potential than hydrogen ions. Hence, silver ions will be deposited in preference to hydrogen ions.
At anode: Silver anode will dissolve to form silver ions in the solution.
Ag → Ag+ + e−
(ii) An aqueous solution of AgNO3? with platinum electrodes.
At cathode: Silver ions have lower discharge potential than hydrogen ions. Hence, silver ions will be deposited in preference to hydrogen ions.
At anode: Hydroxide ions having lower discharge potential will be discharged in preference to nitrate ions. Hydroxide ions will decompose to give oxygen.
4OH− (aq) → 2H2? O (l) + O2? (g) + 4e−
(iii) A dilute solution of H2? SO4? with platinum electrodes.
At cathode: 2H+ + 2e− → H2? (g)
At anode: Hydroxide ions having lower discharge potential will be discharged in preference to sulphate ions. Hydroxide ions will decompose to give oxygen.
4OH− (aq) → 2H2? O (l) + O2? (g) + 4e−
(iv) An aqueous solution of CuCl2? with platinum electrodes
At cathode: Cupric ions will be reduced in preference to protons
Cu2+ + 2e− → Cu
At anode: Chloride ions will be oxidized in preference to hydroxide ions
2Cl− → Cl2? + 2e−
8.7. Suggest a list of substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Substance | Oxidation number of C | Substance | Oxidation number of N |
CH2? Cl2? | 0 | N2? | 0 |
FC≡CF | +1 | N2? O | +1 |
HC≡CH | -1 | N2? H2? | -1 |
CHCl3? , CO | +2 | NO | +2 |
CH3? Cl | -2 | N2? H4? | -2 |
Cl3? C−CCl3? | +3 | N2? O3? | +3 |
H3? C−CH3? | -3 | NH3? | -3 |
CCl4? , CO2? | +4 | NO2? | +4 |
CH4? | -4 | N2? O5? | +5 |
8.11. Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if oxidising agent is in excess. Justify this statement giving three illustrations.
The three examples are:
(i) When excess P4? (reducing agent) reacts with F2? (oxidizing agent), PF3? is produced in which P has +3 oxidation number.
P4? (excess) + F2? → PF3?
But if fluorine is in excess, PF5? is formed in which P has oxidation number of +5.
P4? ? + F2? (excess) → PF5?
(ii) Oxidizing agent is oxygen and reducing agent is K. When excess K reacts with oxygen, K2? O is formed in which oxygen has oxidation number of -2.
4K (excess) + O2? → 2K2? O
But if oxygen is in excess, then K2? O2? is formed in which O has oxidation number of -1.
2K + O2? (excess) → K2? O2?
(iii) The oxidizing agent is oxygen and the reducing agent is C. When an excess of C reacts with oxygen, CO is formed in which C has +2 oxidation number.
C (excess) + O2? → CO
When excess of oxygen is used, CO2? is formed in which C has +4 oxidation number.
C + O2? (excess) → CO2
8.30. Depict the galvanic cell in which the reaction, Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
takes place. Further show:
(i) Which of the electrode is negatively charged.
(ii) The carriers of current in the cell and
(iii) Individual reaction at each electrode. (Advance)
The given redox reaction can be depicted as
Zn (s) + 2Ag+ (aq) → Zn2+ (aq) + 2Ag (s)
Since Zn gets oxidised to Zn2+ ions, and Ag+ gets reduced to Ag metal, therefore,
Oxidation occurs at the zinc electrode and reduction occurs at the silver electrode. Thus, galvanic cell corresponding to the above redox reaction may be depicted as:
Zn|Zn2+ (aq) | Ag+ (aq) | Ag
(i) Zinc electrode is negatively charged because oxidation occurs at the zinc electrode (i.e. electrons accumlulate on the zinc electrode)
(ii) The ions carry current. The electrons flow from Zn to Ag electrode while the current flows from Ag to Zn electrode.
(iii) Reaction occurring at each electrode are:
Zn (s) → Zn2+ (aq) + 2e-
Ag+ (aq) + e- → Ag (s)
8.17. Consider the reactions:
(a) H3PO2(aq) + 4AgNO3(aq) + 2H2O(l) → H3PO4(aq) + 4Ag(s) + 4HNO3(aq)
(b) H3PO2(aq) + 2CuSO4(aq) + 2H2O(l) → H3PO4(aq) + 2Cu(s) + H2SO4(aq)
(c) C6H5CHO(l) + 2[Ag(NH3)2]+(aq) + 3OH–(aq→ C6H5COO–(aq) + 2Ag(s) + 4NH3(aq) + 2H2O(l)
(d) C6H5CHO(l) + 2Cu2+(aq) + 5OH–(aq) → No change observed
What inference do you draw about the behaviour of Ag+ and Cu2+ from these reactions?
Reactions (a) and (b) indicate that H3PO2 (hypophosphorous acid) is a reducing agent and thus reduces both AgNO3 and CuSO4 to Ag and Cu respectively. Conversely, both AgNO3 and CuSO4 act as oxidising agent and thus oxidise H3PO2to H3PO4 (orthophosphoric acid) Reaction (c) suggests that [Ag (NH3)2]+ oxidises C6H5CHO (benzaldehyde) to C6H5COO– (benzoate ion) but reaction (d) indicates that Cu2+ ions cannot oxidise C6H5CHO to C6H5COO–. Therefore, from the above reactions, we conclude that Ag+ ion is a strong deoxidising agent than Cu2+ ion.
8.16. Why does the following reaction occur?
XeO64– (aq) + 2F– (aq) + 6H+(aq) → XeO3(g)+ F2(g) + 3H2O(l)
What conclusion about the compound Na4XeO6 (of which XeO64– is a part) can be drawn from the reaction?
XeO64−? oxidizes F− and F− reduces XeO64−?
Hence, the given reaction occurs.
The oxidation number of Xe decreases from +8 to +6. The oxidation number of F increases from -1 to 0.
Thus, Na4? XeO6? is a stronger oxidising agent than F−.
8.14. Consider the reactions:
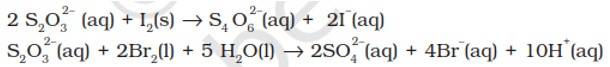
The average O.N. of S in S2O32- is +2 while in S4O62- it is + 2.5. The O.N. of S in SO42- is +6. Since Br2 is a stronger oxidising agent than I2, it oxidises S of S2O32- to a higher oxidation state of +6 and hence forms SO42- ion. I2, however, being weaker oxidising agent oxidises S of S2O32- ion to a lower oxidation of +2.5 in S4O62- ion. It is because of this reason that thiosulphate reacts differently with Br2 and I2.
8.15. Justify giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic acid is the best reductant.
Fluorine oxidizes chloride ion to chlorine, bromide ion to bromine and iodide ion to iodine respectively.
F2? + 2Cl− → 2F− + Cl2?
F2? + 2Br− → 2F− + Br2?
F2? + 2I− → 2F− + I2?
Chlorine oxidizes bromide ion to bromine and iodide ion to iodine.
Cl2? + Br− → 2Cl− + Br2?
Cl2? + I− → 2Cl− + I2?
Bromine oxidizes iodide ion to iodine.
Br2? + I− → 2Br− + I2?
But bromine and chlorine cannot oxidize fluoride to fluorine. Hence, fluorine is the best oxidizing agent amongst the halogens. The decreasing order of the oxidizing power of halogens is F2? >Cl2? >Br2? >I2?
HI and HBr can reduce sulphuric acid to sulphur dioxide but HCl and HF cannot. Thus, HI and HBr are stronger reducing agents than HCl and HF.
2HI+H2? SO4? →I2? +SO2? +2H2? O
2HBr+H2? SO4? →Br2? +SO2? +2H2? O
Iodide ion can reduce Cu (II) to Cu (I) but bromide cannot.
4I−+2Cu2+→Cu2? I2? +I2?
Hence, among the hydrohalic compounds, hydroiodic acid is the best reductant. The reducing power of hydrohalic acids is HF
8.1. Assign oxidation number to the underlined elements in each of the following species:
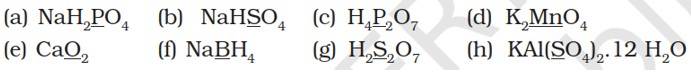
Let x be the oxidation number to the underlined elements in the given species:
(a) NaH2PO4
(+1) + 2 (+1) + x + 4 (-2) = 0
x + 3 – 8 = 0
x = +5
(b) NaHSO4
(+1) + (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(c) H4P2O7
4 (+1) + 2x + 7 (-2) = 0
2x -10 =0
x = +5
(d) K2MnO4
2 (+1) + x + 4 (-2) = 0
x – 6 = 0
x = +6
(e) CaO2
2 + 2x = 0
x = -1
(f) NaBH4
1 + x + 4 (-1) = 0 (Since H is present as hydride ion.)
x = +3
(g) H2S2O7
2 (+1) + 2x + 7 (-2) = 0
x = +6
(h) KAl (SO4)2.12H2O
+1 + 3 + 2x + 8 (-2) + 12 (2 x 1 - 2) = 0
x = +6
8.2. What is the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
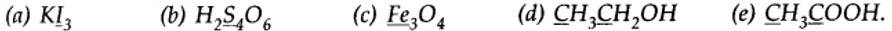
(a) In Kl3, since the oxidation number of K is +1, therefore, the average oxidation number of iodine = -1/3. But the oxidation number cannot be fractional. Therefore, we must consider its structure, K+ [I —I <— I]–. Here, a coordinate bond is formed between I2 molecule and I– ion. The oxidation number of two iodine atoms forming the I2 molecule is zero, while that of iodine forming the coordinate bond is -1. Thus, the oxidation number of the three I atoms, atoms in Kl3 is 0, 0 and -1, respectively.
(b) By conventional method O.N. of S in H2S4O6is calculated as:
2 (+1) +4x + 6) (-2) = 0
Or x = +2.5
But all the four S atoms cannot be in the same oxidation state.
So by chemical bonding method:
The O.N. of each of the S-atoms linked with each other in the middle is zero while that of each of the remaining two S-atoms is +5.
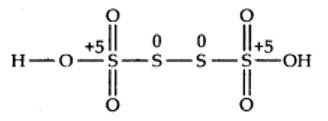
(c) By conventional method O.N. of Fe in Fe3O4 is calculated as:
3x + 4 (-2) = 0 or x = 8/3
By stoichiometry O.N. of Fe in Fe3O4 is +2 and +3.
(d) By conventional method, O.N. of C in CH3CH2OH is calculated as:
2x + 6 (+1) +1 (-2) = 0 or x = -2
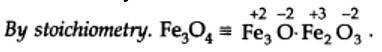
(e) By conventional method, O.N. of C in CH3COOH is calculated as:
2x + 4 - 4 = 0 or x = 0
By chemical bonding method, C2 is attached to three H-atoms (less electronegative than carbon) and one -COOH group (more electronegative than carbon).
Therefore, O.N. of C2 = 3 (+1) + x + 1 (-1) = 0 or x = -2
But C1 is attached to one oxygen atom by a double bond, one OH (-1) and one CH3 (+1) group, therefore, O.N. of C1 = +1 + x+ 1 (-2) + 1 (-1) = 0 or x = +2
8.3. Justify that the following reactions are redox reactions:
(a) CuO (s) + H2(g) → Cu(s) + H2O(g)
(b) Fe2O3(s) +3CO(g) → 2Fe(s) + 3CO2(g)
(c) 4BCl3(g) +3LiAlH4(s) → 2B2H6(g) + 3LiCl(s) + 3AlCl3(s)
(d) 2K(s) +F2(g) → 2K+F–(s)
(e) 4 NH3 (g) + 5 O2 → 4NO (g) + 6H2O (g)
(a) Here, O is removed from CuO, therefore, it is reduced to Cu, while O is added to H2 to form H2O, therefore, it is oxidised. Further, O.N. of Cu decreases from +2 in CuO to 0 in Cu but that of H increases from 0 in H2 to +1 in H20. Therefore, CuO is reduced to Cu but H2 is oxidised to H2O. Thus, this is a redox reaction.
(b) Here O.N. of Fe decreases from +3 in Fe2O3 to 0 in Fe while that of C increases from +2 in CO to +4 in CO2. Further, oxygen is removed from Fe2O3 and added to CO, therefore, Fe2O3 is reduced while CO is oxidised. Thus, this is a redox reaction.
(c) Here, O.N. of B decreases from +3 in BrCl3to -3 in B2H6 while that of H increases from -1 in LiAlH4to +1 in B2H6. Therefore, BCl3 is reduced while LiAlH4 is oxidised. Further, H is added to BCl3 but is removed from LiAlH4, therefore, BC13 is reduced while LiAlH4 is oxidised. Thus, it is a redox reaction.
(d) Here, each K atom as lost one electron to form K+ while F2 has gained two electrons to form two F– ions. Therefore, K is oxidised while F2 is reduced. Thus, it is a redox reaction.
(e) Here, the oxidation number of N increases from –3 in NH3 to +2 in NO. On the other hand, the oxidation number of O2 decreases from 0 in O2 to –2 in NO and H2O i.e., O2 is reduced. Hence, the given reaction is a redox reaction.
8.4. Fluorine reacts with ice and results in the change:
H2O(s) + F2 (g) ——> HF (g) + HOF (g)
Justify that this reaction is a redox reaction.
Writing the O.N. of each atom above its symbol, we have,
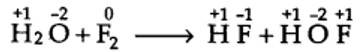
here, the O.N. of F decreases from 0 in F2 to -1 in HF and increases from 0 in F2 to +1 in HOF. Therefore, F2 is both reduced as well as oxidised. Thus, it is a redox reaction and more specifically, it is a disproportionation reaction.
8.5. Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O72- and NO3-. Suggest structure of these compounds. Count for the fallacy.
(a) H2? SO5? by conventional method.
Let x be the oxidation number of S
2 (+1) + x + 5 (−2) = 0
x = +8
+8 oxidation state of S is not possible as S cannot have an oxidation number more than 6. The fallacy is overcome if we calculate the oxidation number from its structure HO−S (O2)−O−O−H.
−1+X+2 (−2)+2 (−1)+1=0
x=+6
(b) Dichromate ion
Let x be the oxidation number of Cr in dichromate ion
2x+7 (−2)=−2
x=+6
Hence the oxidation number of Cr in dichromate ion is +6. This is correct and there is no fallacy.
(c) Nitrate ion, by conventional method
Let x be the oxidation number of N in nitrate ion.
x+3 (−2)=−1
From the structure O−−N+ (O)−O−
x+1 (−1)+1 (−2)+1 (−2)=0
x=+5
Thus there is no fallacy.
8.6. Write formulas for the following compounds:
(a) Mercury (II) chloride, (b) Nickel (II) sulphate, (c) Tin (IV) oxide, (d) Thallium
(I) sulphate, (e) Iron (III) sulphate, (f) Chromium (III) oxide.
(a) HgCl2 (b) NiSO4 (c)SnO2 (d) Tl2SO4 (e) Fe2 (S04)3 (f) Cr2O3.
8.8. While sulphur dioxide and hydrogen peroxide can act as an oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
(i) In SO2, O.N. of S is +4. In principle, S can have a minimum O.N. of -2 and maximum of +6. Therefore, S in SO2 can either decrease or increase its O.N. and hence can act both as an oxidising as well as a reducing agent.
(ii) In H2O2, the O.N. of O is -1. In principle, O can have a minimum O.N. of -2 and maximum of zero (+1 is possible in O2F2 and +2 in OF2). Therefore, O in H2O2 can either decrease its O.N. from -1 to -2 or can increase its O.N. from -1 to zero. Therefore, H2O2 acts both as an oxidising as well as a reducing agent.
(iii) In O3, the O.N. of O is zero. It can only decrease its O.N. from zero to -1 or -2, but cannot increase to +2. Therefore, O3 acts only as an oxidant.
(iv) In HNO3, O.N. of N is +5 which is maximum. Therefore, it can only decrease its O.N. and hence it acts as an oxidant only.
8.9. Consider the reactions:
(a) 6CO2(g) + 6H2O(l) →C6H12O6(s) + 6O2(g)
(b) O3(g) + H2O2(l) →H2O(l) + 2O2(g)
Why it is more appropriate to write these reactions as:
(a) 6CO2(g) + 12H2O(l) →C6H12O6(s) + 6H2O(l) + 6O2(g)
(b) O3(g) + H2O2 (l) →H2O(l) + O2(g) + O2(g)
Also suggest a technique to investigate the path of above (a) and (b) redox reactions.
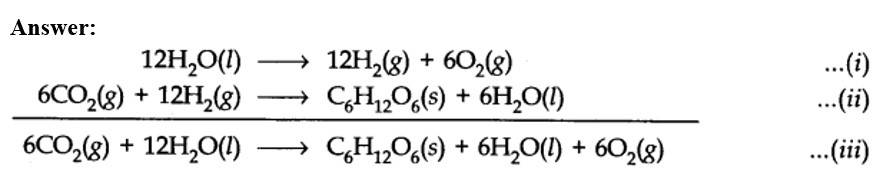
(a) Therefore, it is more appropriate to write the equation for photosynthesis as (iii) because it emphasises that 12H2O are used per molecule of carbohydrate formed and 6H2O are produced during the process.
(b) The purpose of writing O2 two times suggests that O2 is being obtained from each of the two reactants.
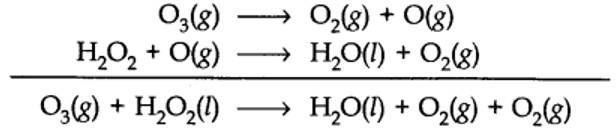
(a) or by using H2O218 or O318in reaction (b).
8.13. Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions.
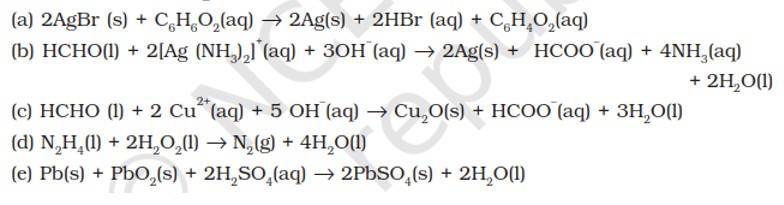
Substance oxidised | Substance reduced | Oxidising agent | Reducing agent |
(a) C6H12O6 | AgBr | C6H12O6 | |
(b) HCHO | [Ag (NH3)2]+ | [Ag (NH3)2]+ | HCHO |
(c) HCHO | Cu2+ | HCHO | |
(d) N2H4 | H2O2 | N2H4 | |
(e) Pb | PbO2 | Pb |
8.20. What sorts of information can you draw from the following reaction?
(CN)2(g) + 2OH–(aq) → CN–(aq) + CNO–(aq) + H2O(l)
Let x be the O.N. of C.
O.N. of C in cyanogen, (CN)2 = 2 (x – 3) = 0 or x = +3
O.N. of C in cyanide ion, CN- = x – 3 = -1 or x = +2
O.N. of C in cyanate ion, CNO =x-3-2 = -l or x: = +4
The four information about the reaction are:
(i) The reaction involves decomposition of cyanogen, (CN)2 in the alkaline medium to cyanide ion, CN and cyanate ion, CNO–.
(ii) The O.N. of C decreases from +3 in (CN)2 to +2 in CN–ion and increases from +3 in (CN)2 to +4 in CNO– ion. Thus, cyanogen is simultaneously reduced to cyanide ion and oxidised to cyanate ion.
(iii) It is an example of a redox reaction in general and a disproportionation reaction in particular.
(iv) Cyanogen is a pseudohalogen (behaves like halogens) while cyanide ion is a pseudohalide ion (behaves like halide ion).
8.21. The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+, MnO2 and H+ ion. Write a balanced ionic equation for the reaction.
The unbalanced chemical reaction is:
Mn3+ (aq) → Mn2+ (aq) + MnO2? (s) + H+ (aq)
The oxidation half reaction is,
Mn3+ (aq) → MnO2? (s).
To balance oxidation number, one electron is added on R.H.S.
Mn3+ (aq) → MnO2? (s) + e−
4 protons are added to balance the charge.
Mn3+ (aq) → MnO2? (s) + 4H+ (aq) + e−
2 water molecules are added to balance O atoms.
The reduction half reaction is Mn3+ (aq) → Mn2+ (aq).
An electron is added to balance oxidation number.
Mn3+ (aq) + e− → Mn2+ (aq)
Two half-cell reactions are added to obtain balanced chemical equation.
2Mn3+ (aq) + 2H2? O (l) → Mn2+ (aq) + MnO2? (s) + 4H+ (aq)
8.22. Consider the elements:
Cs, Ne, I, F
(a) Identify the element that exhibits negative oxidation state.
(b) Identify the element that exhibits positive oxidation state.
(c) Identify the element that exhibits both positive and negative oxidation states.
(d) Identify the element which neither exhibits negative nor does the positive oxidation state.
(a) F. Fluorine being the most electronegative element shows only a -ve oxidation state of -1.
(b) Cs. Alkali metals because of the presence of a single electron in the valence shell, exhibit an oxidation state of +1.
(c) I. Because of the presence of seven electrons in the valence shell, I shows an oxidation state of -1 (in compounds of I with more electropositive elements such as H, Na, K, Ca, etc.) or an oxidation state of +1 compounds of I with more electronegative elements, i.e., O, F, etc.) and because of the presence of d-orbitals it also exhibits +ve oxidation states of +3, +5 and +7.
(d) Ne. It is an inert gas (with high ionization enthalpy and high positive electron gain enthalpy) and hence it neither exhibits -ve nor +ve oxidation states.
8.23. Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The balanced chemical reaction for the redox reaction between chlorine and sulphur dioxide is:
Cl2? + SO2? + 2H2? O → 2Cl− + SO42− ? + 4H+.
8.24. Refer to the periodic table given in your book and now answer the following questions.
(a) Select the possible non-metals that can show disproportionation reaction.
(b) Select three metals that show disproportionation reaction.
(a) Phosphorous, chlorine and sulphur are the non metals which can show disproportionation reaction. The reactions shown are:
P4? + 3OH? + 3H2? O? PH3? + 3H2? PO2?
? Cl2? + 2OH? ? Cl? + ClO? + H2? O
S8? +12OH? ?4S2? +2S2? O32? ? +6H2? O
(b) Copper, gallium and indium are the metals that show disproportionation reaction.
The reactions are shown below.
2Cu+? Cu2+ + Cu
3Ga+? Ga3+ + 2Ga
3In+? In3+ + 2In
8.25. In Ostwald’s process for the manufacture of nitric add, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum Weight of nitric oxide that can be obtained starting only with 10.0 g of ammonia and 20.0 g of oxygen?
The balanced equation for the reaction is:
4NH3? (g)+5O2? (g)→4NO (g)+6H2? O (g)
The molar masses of ammonia and oxygen are 17 g/mol and 32 g/mol respectively.
68 g of NH3 will react with O2 = 160 g.
Therefore, 10 g of NH3 will react with O2 = 160/68 x 10 g = 23.6 g
But the amount of O2 which is actually available is 20.0 g which is less than the amount which is needed. Therefore, O2 is the limiting reagent and hence calculations must be based upon the amount of O2 taken and not on the amount of NH3 taken. From the equation,
160 g of O2 produce NO = 120 g
Therefore, 20 g of O2 will produce NO =120/160 x 20 = 15 g
8.28. Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn.
Based on the relative positions of these metals in the activity series, the correct order is Mg, Al, Zn, Fe, Cu.
Assertion and Reason:
Directions:
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
8.31. Assertion: Standard hydrogen electrode is called as reversible electrode.
Reason: It can react both as an anode as well a cathode in an electrochemical cell.
Answer: (a) A standard hydrogen electrode is called a reversible electrode because it can react both as anode as well as cathode in an electrochemical cell.
8.33. Assertion: The highest value of oxidation number exhibited by an atom of an element generally increases across the period in the periodic table.
Reason: The oxidation number denotes theoxidation state of an element in a compound.
(b) Both are correct statements but reason is not correct for the assertion. The highest oxidation number of a representative element is the group number for the first two groups and the group number minus 10 (following the long form of periodic table) for the other groups.
8.34. Assertion: While H2O2 can only act as oxidising agent in their reactions.
Reason: In H2O2 oxidation number of O is -1 and can vary from 0 to -2
(d) In H2O2 oxidation number of O = -1 and can vary from 0 to -2 (+2 is possible in OF2). The oxidation number can decrease or increase, because of this H2O2 can act both asoxidising and reducing agent.
8.35. Assertion: ClO4– does not show disproportionation reaction.
Reason: In ClO4–, chlorine is in minimum oxidation state of +1.
(c) In ClO4–, chlorine is in maximum oxidation state of +7. So, it does not show the disproportionation reaction.
8.38. Choose the incorrect statement.
(a) HNO3 acts only as an oxidising agent while HNO3 can act both as reducing and oxidising agent.
(b) ClO4– does not show disproportionation reaction.
(c) Ozone acts as an oxidising agent.
(d) None of the above
(d) None of the above
8.39. Choose the incorrect statement.
(a) A redox couple consists of oxidised and reduced form of the same substance taking part in the oxidation or reduction half reaction.
(b) Oxidation involves gain of one or more electrons by a species during a reaction.
(c) Half reactions that involve loss of electrons are called oxidation reactions.
(d) Half reactions that involve gain of electrons are called reduction reactions.
(b) Oxidation involves gain of one or more electrons by a species during a reaction.
Questions and Answers:
8.41. Define electrochemical cell. How will you identify cathode and anode in an electrochemical cell?
Electrochemical cell is a device in which the redox reaction is carried indirectly and the decrease in free energy appears as electrical energy.
At cathode there is gain of electrons.
At anode there is loss of electrons.
In electrochemical cell anode is written on L.H.S while cathode is written on R.H.S.
8.42. What is meant by electrochemical series? What are the characteristics of electrochemical series?
Electrochemical series is the series of elements in which elements are arranged in decreasing order of their reduction potential.
Reducing power goes on increasing whereas oxidising power goes on decreasing down the series.
8.43. Name the different types of redox reactions.
(a) Combination reactions (b) Decomposition reactions (c) displacement reactions (d) Disproportionation reactions.
8.44. What are the methods of balancing redox reactions.
Two methods are used to balance chemical equations for redox processes. One of these methods is based on the change in the oxidation number of reducing agent and the oxidising agent (i.e. oxidation number method) and the other method is based on splitting the redox reaction into two half reactions — one involving oxidation and the other involving reduction (half reaction method). Both these methods are in use and the choice of their use rests with the individual using them.
8.45. What do you understand by Standard Electrode Potential? Explain.
The potential associated with each electrode is known as electrode potential. If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further there action is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential. By convention, the standard electrode potential (E? ) of hydrogen electrode is 0.00 volts. The electrode potential value for each electrode process is a measure of the relative tendency of the active species in the process to remainin the oxidised/reduced form. A negative E means that the redox couple is a stronger reducing agent than the H+/H2 couple. A positive E? means that the redox couple is a weaker reducing agent than the H+/H2 couple.
32. Assertion: Na+ ion has an oxidation number of +1, Mg2+ ion, +2, Fe3+ ion, +3, Cl– ion, –1, O2– ion, –2; and so on.
Reason: For ions composed of only one atom, the oxidation number is equal to the charge on the ion.
Kindly go through the solution
(a)
36. Oxidation number ofS in S2O8-2 is
(a) -2 (b) -6 (c) +2 (d) +6
Kindly go through the solution
(d) +6
37. Which element is considered to have oxidation number of +3 in all its compounds?
(a) Be (b) Li (c) Al (d) Sc
Kindly go through the solution
(c) Al
40. Best reducing agent is:
(a) He (b) Be (c) Li (d) Al
Kindly go through the solution
(c) Li
Redox Reaction Additional Questions
Check some additional questions on oxidation and reduction class 11 below. Students must prepare the class 11 redox reactions notes on important points for quick revision. Practice the question to secure good marks in exam.
| Assertion and Reason: Directions: (a) Assertion and reason both are correct statements and reason is correct explanation for assertion. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion. (c) Assertion is correct statement but reason is wrong statement. (d) Assertion is wrong statement but reason is correct statement.
8.1. Assertion: Standard hydrogen electrode is called as reversible electrode. |
| Reason: It can react both as an anode as well a cathode in an electrochemical cell. |
| 8.2. Assertion: Na+ ion has an oxidation number of +1, Mg2+ ion, +2, Fe3+ ion, +3, Cl– ion, –1, O2– ion, –2; and so on. Reason: For ions composed of only one atom, the oxidation number is equal to the charge on the ion. |
| Answer: (a) |
| 8.3. Assertion: The highest value of oxidation number exhibited by an atom of an element generally increases across the period in the periodic table. Reason: The oxidation number denotes theoxidation state of an element in a compound. |
| Answer: (b)Both are correct statements but reason is not correct for the assertion. The highest oxidation number of a representative element is the group number for the first two groups and the group number minus 10 (following the long form of periodic table) for the other groups. |
| 8.4. Assertion: While H2O2 can only act as oxidising agent in their reactions. |
| Answer: (d) In H2O2 oxidation number of O = -1 and can vary from 0 to -2 (+2 is possible in OF2). The oxidation number can decrease or increase, because of this H2O2 can act both asoxidising and reducing agent. |
| 8.5. Assertion: ClO4– does not show disproportionation reaction. Reason: In ClO4–, chlorine is in minimum oxidation state of +1. |
| Answer: (c)In ClO4–,chlorine is in maximum oxidation state of +7. So, it does not show the disproportionation reaction. |
| MCQs
8.1. oxidation number ofS in S2O8-2 is (a) -2 (b) -6 (c) +2 (d) +6 |
| Answer: (d) +6 |
| 8.2. Which element is considered to have oxidation number of +3 in all its compounds? (a) Be (b) Li (c) Al (d) Sc |
| Answer: (c) Al |
| 8.3. Choose the incorrect statement. (d) none of the above |
| Answer: (d) none of the above |
| 8.4. Choose the incorrect statement. (a) A redox couple consists of oxidised and reduced form of the same substance taking part in the oxidation or reduction half reaction. (b) Oxidation involves gain of one or more electrons by a species during a reaction. (c) half reactions that involve loss of electrons are called oxidation reactions. (d) half reactions that involve gain of electrons are called reduction reactions. |
| Answer: (b)Oxidation involves gain of one or more electrons by a species during a reaction. |
| 8.5. Best reducing agent is: |
| Answer: (c) Li |
| Questions and Answers: 8.1. Define electrochemical cell. How will you identify cathode and anode in an electrochemical cell? |
| Answer: Electrochemical cell is a device in which the redox reaction is carried indirectly and the decrease in free energy appears as electrical energy. At cathode there is gain of electrons. |
| 8.2. What is meant by electrochemical series? What are the characteristics of electrochemical series? |
| Answer: Electrochemical series is the series of elements in which elements are arranged in decreasing order of their reduction potential. |
| 8.3. Name the different types of redox reactions. |
| Answer: (a) Combination reactions (b) Decomposition reactions (c) displacement reactions (d) Disproportionation reactions. |
| 8.4. What are the methods of balancing redox reactions. |
| Answer: Two methods are used to balance chemical equations for redox processes. One of these methods is based on the change in the oxidation number of reducing agent and the oxidising agent (i.e. oxidation number method) and the other method is based on splitting the redox reaction into two half reactions — one involving oxidation and the other involving reduction (half reaction method). Both these methods are in use and the choice of their use rests with the individual using them. |
| 8.5. What do you understand by Standard Electrode Potential? Explain. |
| Answer: The potential associated with each electrode is known as electrode potential. If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further there action is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential. By convention, the standard electrode potential (Eϴ) of hydrogen electrode is 0.00 volts. The electrode potential value for each electrode process is a measure of the relative tendency of the active species in the process to remainin the oxidised/reduced form. A negative E means that the redox couple is a stronger reducing agent than the H+/H2 couple. A positive Eϴ means that the redox couple is a weaker reducing agent than the H+/H2 couple. |
Redox Reaction - FAQs
The following are the FAQs on Redox Reaction:
Commonly asked questions
Define redox reaction according to Class 11 Chemistry.
In a chemical reaction when electrons transfer simultaneously between substances, it is called as the redox reaction. In redox reaction, one substance gets oxidized by losing electrons and another substance reduces by gaining the electrons. These two processes together occurs in a redox reaction.
Name the different types of redox reactions.
There are four types of redox reactions. These are combination, Decomposition, Displacement, and Disproportionation.
In the combination redox reaction, two or more substances form a single product, in decomposition reactions, a compound breaks down into two or more substances. When one element in a compound replaces another, it is called displacement reactions. When simultaneously, one substance is oxidized and reduced, the reaction is known as the disproportionation redox reaction.
Why redox reactions are essential?
The redox reactions are significant for some of the basic functions of life such as respiration, photosynthesis, corrosion or rusting and combustion.
Which are the most common applications of the redox reaction?
The applications of redox reactions include in cellular respiration, and batteries. In industries, these are used in the chemical production, metal extraction and water treatment.
What is Redox Reaction Class 11 Chemistry weightage in NEET and JEE Main exams?
In NEET exam, there can be 1 to 2 questions from redox reactions, carrying 4-8 marks. The weightage is around 2% to 4%. In JEE Main, the weightage of this chapter is from 3.3% to 4-6%. You can expect nearly 1 to 2 questions from this chapter.
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
