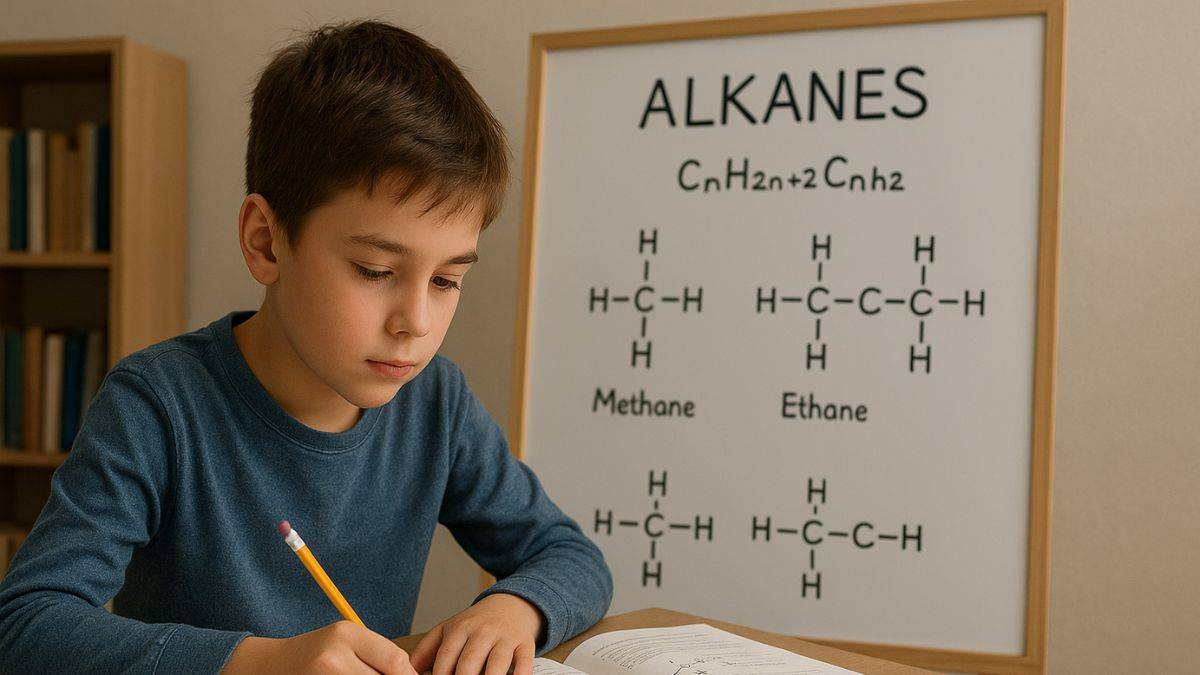Next topic which we come onto in organic chemistry is alkanes, known as the simplest type of compounds. Alkanes form the backbone of any hydrocarbons and help in formation of new bonds which in turn leads to some more complex products such as alkenes and alkynes. Alkanes are known as saturated hydrocarbons because each and every carbon atom can bond to multiple hydrogen atoms. They are naturally found in fuels like natural gas, petroleum and coal.
If you wish to prepare thoroughly for JEE MAINS, go through this article to help strengthen your core base and understanding of crucial concepts of hydrocarbons.
| NCERT Class 11 notes | |
| Chemistry Class 11 NCERT notes |
- What are Alkanes?
- Structure of Alkanes
- Nomenclature of Alkanes
- Properties of Alkanes
- Preparation of Alkanes
- Important Graphs
- Isomerization
- Class 11 Chemistry Notes: Chapter Wise
- Class 11 Chemistry NCERT Solutions: Chapter Wise
What are Alkanes?
Let us know about the alkanes, the simplest type of compounds to exist in organic chemistry. These are saturated hydrocarbons made up of entirely carbon and hydrogen atoms. They can also be called paraffins, which is derived from the latin word affins meaning low activity (single bond between the carbon atoms).
The general formula for alkanes is:
where n = number of carbon atoms
Some common examples of Alkanes include
- methane (CH4)
- ethane (C2H6)
- propane (C3H8)
- butane (C4H10)
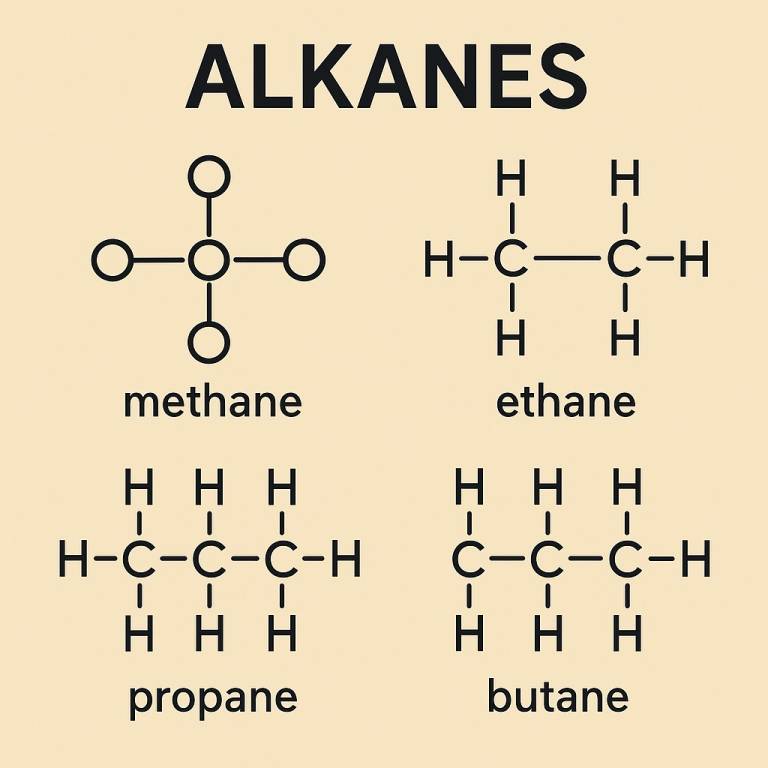
Structure of Alkanes
Now talking about the structure of alkanes, they have a simple structure where only carbon and hydrogen atoms can react with each other and each carbon atom can form 4 bonds. Their structure follows the sp3 hybridization (tetrahedral shape) where each bond forms an angle of 109.5. Each and every bond in alkanes is a sigma bond.
Generally, Alkanes can be:
- Straight-Chain: Like butane , where carbons are in a single line.
- Branched-Chain: Like isobutane , where the chain branches off.
- Cycloalkanes:
In these hydrocarbons, the shape of atoms is similar to a ring like structure (also known as ring alkanes). General formula for cycloalkanes is:
CnH2n
Examples include cyclobutane, cyclopropane, cyclohexane, etc.
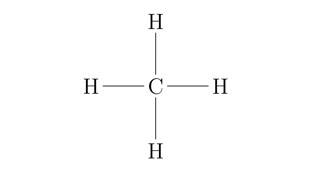
Methane
is known to be the simplest alkane with one carbon atom connected to four hydrogen atoms.
Nomenclature of Alkanes
There are a certain set of rules defined for naming the alkanes. These rules are set by the IUPAC nomenclature of organic compounds (International Union of Pure and Applied Chemistry) to identify the structure and name it accordingly.
Here’s a breakdown of the steps involved in the procedure of naming the alkane:
- First step is to identify the longest chain. The number of carbon atoms will determine what shall be the name of the chain, and these are depicted as follows:
1 C → Methane
2 C → Ethane
3 C → Propane
4 C → Butane
5 C → Pentane
6 C → Hexane
7 C → Heptane
8 C → Octane
9 C → Nonane
10 C → Decane
- Then, start numbering the chain from the end to the nearest branch to ensure the lowest possible numbers for substituents
- Start identifying the substituents and name them accordingly
- After that, assign the numbers to each substituent
- Next step is to assign prefixes. Di for 2, tri for 3, and tetra for 4.
- Finally, arrange all the steps in alphabetical order and here is your alkane name.
Properties of Alkanes
Alkanes possess some unique properties due to their single bond structure. Some of these are as follows:
1. Physical Properties:
- Alkanes cannot dissolve in water. Instead, they do merge with some other organic solvents such as benzene. This is called having a non-polar nature.
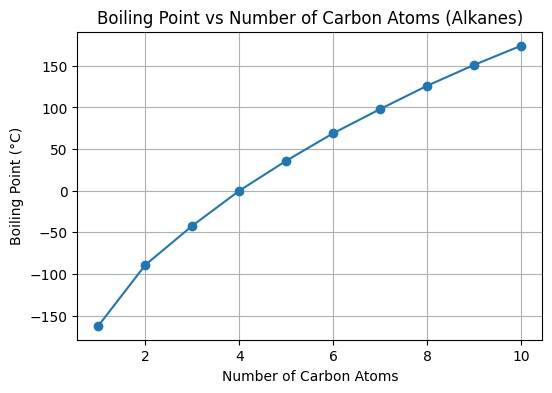
- Boiling points of alkanes can increase with change in the molecular size (e.g., methane: -161.5rC, ethane: - 88.6 re C). This happens because of the strong van der Waals forces.
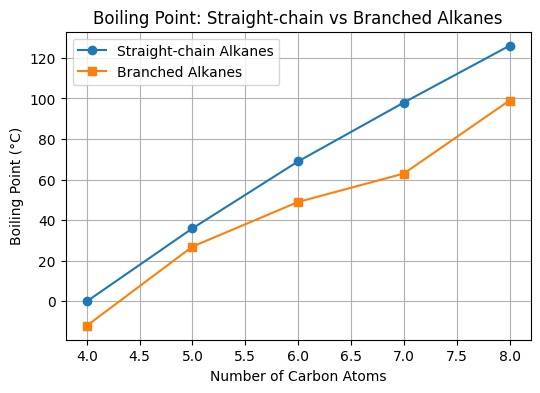
- Due to less surface area, Branched alkanes have a comparatively lower boiling point than straight-chained ones.
- C1 - C4 are gases, C5 - C17 are liquids, C18 and above all are waxes.
2. Chemical Properties:
Alkanes are not very reactive due to the single carbon carbon bond. (saturated). Still, they can undergo some other reactions such as:
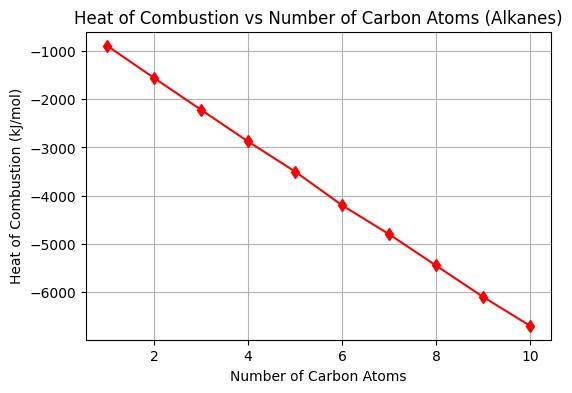
- Combustion: Alkanes burn in oxygen to form and , releasing energy:
- Substitution Reaction: Alkanes react with halogens (like ) in sunlight via a free radical mechanism known as halogenation:
Preparation of Alkanes
There are various methods used for preparation of alkenes, out of which some commons ones are mentioned as follows:
- Hydrogenation:
If we add H2 and alkenes in the presence of a catalyst, the product of the reaction will be alkane.
For example:
RCH=CH2+H2Ni/Pt/Pd,ΔRCH2CH3
- From Alkyl Halides:
On Reduction using Zinc and Hydrochloric acids, alkyl halides can turn into alkanes.
For example:
CH3CH2Br+2[H]Zn/HClCH3CH3+HBr
Alkyl Halides can also react with sodium metal in dry ether to form higher alkanes. This is called Wurtz reaction.
For Example:
2CH3Br+2Nadry etherCH3–CH3+2NaBr
- From Carboxylic Acids:
There are two very important terms under this technique which the JEE MAIN aspirants must clearly go through. Let’s dive into their detailed aspects:
- Decarboxylation: This is the process of removing COOH from a compound, which releases CO2 and results in the formation of an alkane.
Example: CH3COONa+NaOHCaOΔCH4+Na2CO3
- Kolbe’s Electrolysis: This is an electrochemical method where electrolysis of an aqueous solution containing sodium and potassium salts is done to obtain an alkane.
Example: 2CH3COONa electrolysis C2H6+2CO2+2NaOH
Important Graphs
Refer to these graphs given below for detailed understanding of the relations in hydrocrabons:
Figure no. 1: Boiling Point as compared to Carbon Atoms
Figure No. 2: Boiling Point of Straight vs Bracnched Chain Alkanes
Figure No. 3: Heat of Combustion
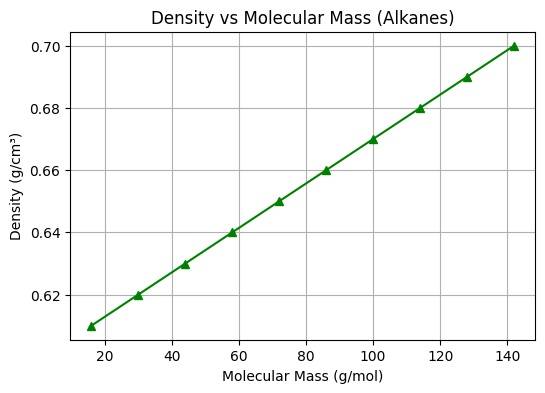
Figure No. 4: Density vs Molecular Mass
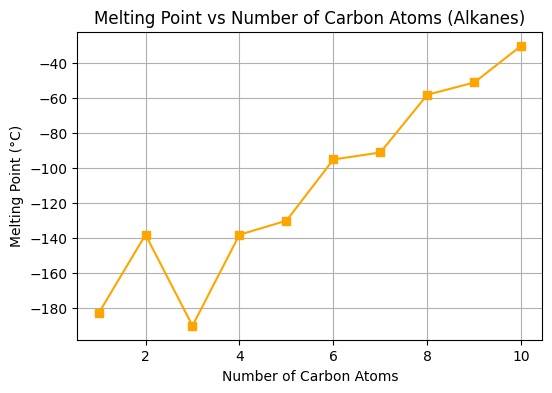
Figure No. 5: Melting Point vs Number of Carbon Atoms
Isomerization
Isomerization, or isomerism, is the process of converting a straight chained alkane into a branch chain alkane using the same molecular formula. Alkanes do not show any functional group isomerism due to their single bonds and only show structural isomerism.
Alkanes can also twist around their C-C single bonds, leading to different shapes called conformations. For ethane , the two main forms will be:
- Eclipsed: Hydrogens on adjacent carbons are lined up (less stable due to repulsion).
- Staggered: Hydrogens are as far apart as possible (more stable).
Click Here: Chemistry NCERT Chapter 9: Hydrocarbons
Isomerism in alkanes can be categorized into two types:
- Chain Isomerism: There can exist different arrangements of carbon atoms in the same chain. Example: n-butane (CH₃CH₂CH₂CH₃) and isobutane (CH₃CH(CH₃)CH₃).
- Conformational Isomerism: Here, there is a rotation possible around the C-C bond. Example: Ethane: staggered (more stable) & eclipsed (less stable).
Class 11 Chemistry Notes: Chapter Wise
Class 11 Chemistry NCERT Solutions: Chapter Wise
Commonly asked questions
Why are alkanes comparatively less reactive as compared to alkenes or alkynes?
This happens due to the fact that they possess strong C-C and H-H bonds which are non-polar sigma bonds and cannot be broken easily. Also, they lack polar functional groups and are saturated hydrocarbons. These properties combined make them less reactive overall.
How are alkanes used in real life?
Here are the important applications of alkanes in our everyday lives:
- Fuel in Vehicles
- Heating and Cooking
- Lubrication
- Power Generation
- Wax and Solvents
What is aromatization?
Aromatization is the procedure of converting an aliphatic compound into a non aromatic compound using a catalyst. This is usually done through the pathwya of techniques like dehydrogenation where a non aromatic compound is converted into an aromatic compound. Examples: converting cyclohexane to benzene, androgens to estrogens, etc. The final product always contains a benzene-like ring structure which follows Hückel's rule.
Chemistry Hydrocarbon Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics