Let us now jump onto the last class of hydrocarbons in organic chemistry. After alkanes and alkenes, alkynes are the third type of compounds which are unsaturated in nature i.e. they consist of two or more than two bonds between their carbon atoms. Alkynes are considered to be the most reactive hydrocarbons since the multiple bonds possess high electron density, making them responsive to various types of chemical reactions.
This article covers various important concpets of alkynes such as nomenclature, isomerism, structure, properties etc. suitable for JEE MAINS.
Also Read:
| NCERT Class 11 notes | |
| Chemistry Class 11 NCERT notes |
- What are Alkynes?
- Structure of Alkynes
- Nomenclature of Alkynes
- Preparation of Alkynes
- Properties of Alkynes
- Isomerism in Alkynes
- Class 11 Chemistry Notes: Chapter Wise
- Class 11 Chemistry NCERT Solutions
What are Alkynes?
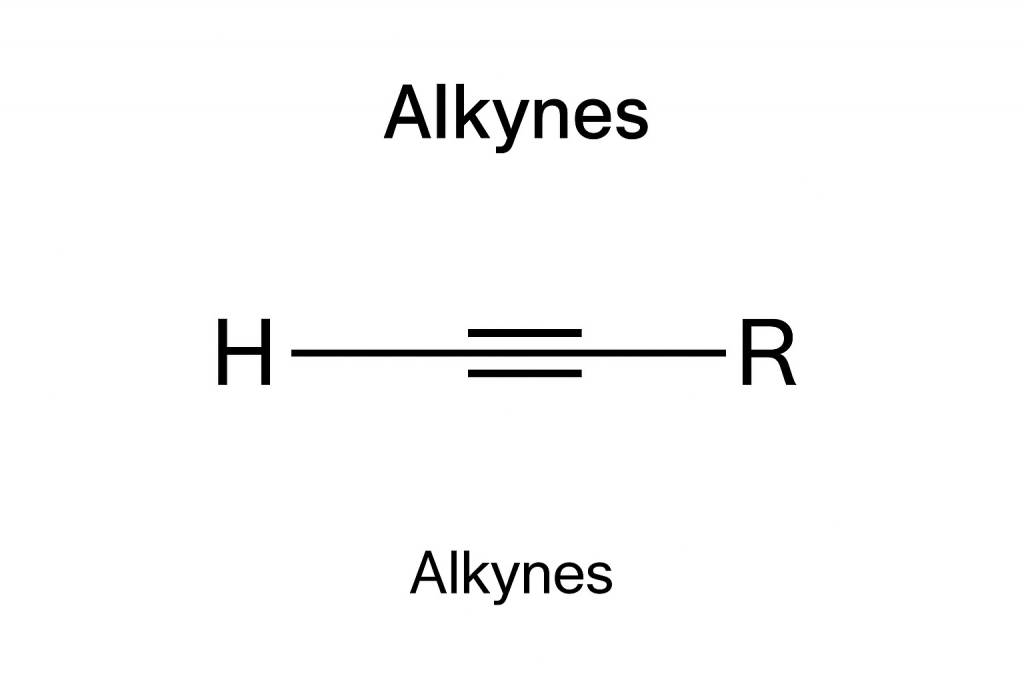
Alkynes have atleast one triple bond between their two carbon atoms (C≡C). Unlike alkanes and alkenes, these unsaturated hydrocarbons are naturally very rare to find and occur in some plants, microorganisms, fossil fuels, minerals etc. Due to the presence of their carbon carbon triple bonds, they are widely known for their high reactivity about which we will briefly study in the article further. The simplest known alkyne to exist is C2H2, scientifically known as Ethyne/acetylene.
The general formula for alkynes is:
Where n = number of carbon atoms.
Some common examples of alkynes are propyne (C3H4), butyne (C4H6), pentyne (C5H8) etc.
Structure of Alkynes
Now let us talk about the structure of alkynes which makes them distinguish others in terms of stability and reactivity mechanisms. Their structure consists of minimum 1 triple bond between their carbon atoms.
The CC triple bond in alkynes consists of one sigma (σ) bond and two pi (π) bonds. The carbon atoms in the triple bond are sp hybridized, which means that they form a linear geometrical shape with bond angles of 180 degrees each. The sigma bond is formed by the head to head overlapping of hybridized sp orbitals. On the other hand, pi bonds are formed due to the sideways overlapping of the unhybridized p orbitals.
For example, in ethyne ( ):
Both the carbon atoms have two bonds, one towards the carbon atom and other towards the hydrogen atom. Both the carbon atoms have one bond to each other respectively, thereby forming a straight line.
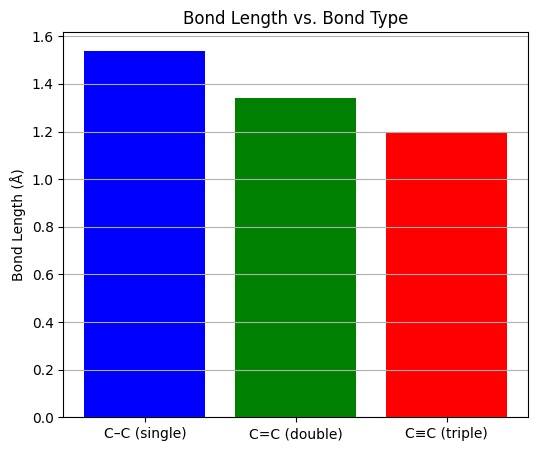
The carbon carbon bond found is alkynes is stronger compared to those of alkanes and alkenes. This triple bond is also shorter in length which leads to the alkynes becoming even more reactive than alkenes and alkanes due to the bonds becoming more weaker so that they can be broken easily.
Nomenclature of Alkynes
The IUPAC (International Union of Pure and Applied Chemistry) has set some certain rules and regulations known as the nomenclature of organic compounds which are to be followed and used to identify and name the alkynes. These rules help in deifining a unqiue name for each and every possible alkyne.
The steps determined by these rules are mentioned as follows for reference:
- What you have to do first is to find the longest carbon chain that includes the triple bond which is known as the parent chain. Use the alkane name but just change -ane to -yne (e.g., ethyne, propyne).
- Now number the chain to give the triple bond the lowest possible number.
- If there are branches too (alkyl groups like methyl, ), name and number them accordingly.
- Suppose if there are multiple triple bonds. In such scenarios you can use prefixes like di-, tri- (e.g., buta-1,3-diyne for ).
For eg, is propyne:
- Longest chain with triple bond: 3 carbons (prop-).
- Triple bond is at position 1.
- So, its name will be propyne.
Note: When both double (ene) and triple (yne) bonds are present, use the suffix “enyne”. An if there are multiple triple bonds, use “diyne” for 2 bonds and “triyne” for 3 bonds. Yes you guessed it correct, naming alkynes is actually a bit more complex as compared to alkanes and alkenes.
Preparation of Alkynes
Alkynes can be prepared in the lab using several methods, some of which you should definitely know. The temperature of solutions is changed to observe how the reaction gets affected due to this (arrhenius equation). Some core techniques of preparing alkynes are explained below:
1. Dehydrohalogenation of Vicinal Dihalides:
Dehydrohalogenation is the process of preparing alkynes from Vicinal Dihalides. Use reagents like alcoholic KOH/NaNH2 and remove 2 HX molecules from the equation to gain the solution.
Example: C₂H₄Br₂ + 2 KOH (alc.) → C₂H₂ + 2 KBr + 2 H₂O
2. From Calcium Carbide (Industrial Method)
CaC2 can react with water to produce ethyne, which is a technique used by various industries for mass production of ethyne/acetylene.
Equation can be presented as: CaC2+2H2O→C2H2+Ca(OH)2
3. From Tetrahalides
Heating tetrahalides with zinc or a strong base can also result in the production of alkynes.
Example: CHBr2-CHBr2Zn/CH3COOHCH≡CH
4. Double Dehydrohalogenation:
This is considered as an elimination method where alkynes can be obtained from a dihalide. here you have to perform the procedure of Dehydrohalogenation twice in order to obtain a dihalide solution.
Example:
CH₃–CHBr–CH₂Br + 2 NaNH₂ → CH₃–C≡CH + 2 NaBr + 2 NH₃
Properties of Alkynes
Alkynes possess some unique propertiesdue to their triple bond. Let’s break them down:
Physical Properties:
- Not dissolving in water and merging instead in organic solvents (like benzene) is called having a non-polar nature, which is found in alkynes.
- Also, their boiling points can increase with change in the molecular size due to the presence of a stronger van der Waals forces. (e.g., ethyne: -84řC, propyne: 23řC)
- They are gases , liquids ( ), or solids ( and above) at room temperature.
Chemical Properties:
Alkynes are very reactive because of their CC triple bond. Here are some key reactions:
- Addition Reactions: In such reactions, the triple bond is broken to form new compounds:
Ethyne first forms ethene and then ethane (hydrogenation).
Ethyne forms 1,1,2,2-tetrabromoethane.
- Addition of HX: Through Markovnikovs rule, Alkynes can comfortably respond to mixing up with hydrogen halides:
Ethyne then forms 1,1-dibromoethane after vinyl bromide,.
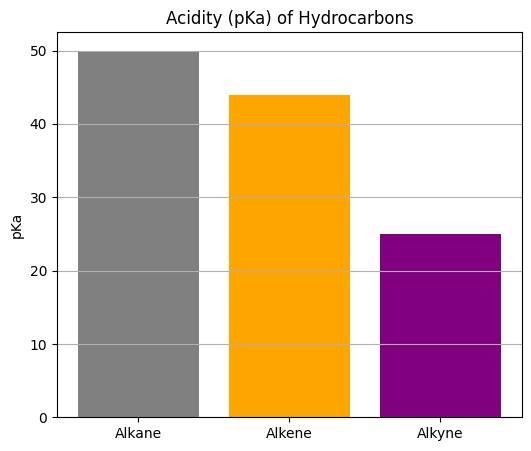
- Acidic Nature: Terminal alkynes are weakly acidic in nature since the hydrogen atom can be easily removed by a strong base like NaNH2:
This leads to the formation sodium acetylide, which can cause reactions further.
- Combustion: Alkynes burn in oxygen to form and :
Isomerism in Alkynes
isomerism is a phenomenon in chemistry where two or more than two compounds show the same molecular formula but the difference lies in the arrangement of their carbon atoms. Alkynes mainly show two types of isomerism:
- Structural Isomerism: These isomers exhibit the same formula in different structures. For example, can be but-1-yne or buta-1,3-diyne (HCC-CCH).
- Position Isomerism: Here, the position of the triple bond changes (either chain or branched), e.g., but-1-yne but-2-yne ( ).
*Note: Unlike alkenes, alkynes don’t show geometrical (cis-trans) isomerism because of their linear shape and the bond angle of 180 degrees (due to the sp hybridization) which allows free rotation.
Click Here: Chemistry NCERT Chapter 9: Hydrocarbons
Class 11 Chemistry Notes: Chapter Wise
The notes given below are specifically designed keeping in mind the syllabus of JEE MAINS which will assist the candidates in their preparation:
| S.No. | Chapter Name |
|---|---|
| 1 | Some Basic Concepts of Chemistry Notes |
| 2 | Structure of Atom Notes |
| 3 | Classification of Elements and Periodicity in Properties Notes |
| 4 | Chemical Bonding and Molecular Structure Notes |
| 5 | States of Matter: Gases and Liquids Notes |
| 6 | Thermodynamics Notes |
| 7 | Equilibrium Notes |
| 8 | Redox Reactions Notes |
| 9 | The s-Block Element Notes |
| 10 | The p-Block Element Notes |
| 11 | Organic Chemistry - Some Basic Principles and Techniques Notes |
| 12 | Hydrocarbons Notes |
| 13 | Environmental Chemistry Notes |
Class 11 Chemistry NCERT Solutions
Commonly asked questions
Does bromine water test work for alkynes?
Bromine water test is a technique used for the identification of alkynes. Alkynes on coming in contact with bromine turn them colourless from reddish brown color. This happens because of the addition reaction which takes place due to the triple carbon bonds of alkynes.
What are terminal and non terminal alkynes?
Terminal alkynes and Non Terminal Alkynes are both variants of alkynes, but the difference lies in the position of the triple carbon bond. Terminal Alkynes have the triple carbon bond at the end of the chain, such as ethyne and propyne. On the other hand, Non Terminal Alkynes have their triple carbon bond in the middle of the chain. Example: 2-Butyene.
What is tautomerism?
Tautomerism is a dynamic equilibrium between 2 isomers, also known as tautomers, where a compound exists in 2 different structures. The most common form is keto enol tautomerism. It is mainly of two types:
- Keto Form: Contains a C=O (carbonyl) group
- Enol Form: Contains a C=C double bond and an –OH group
Chemistry Hydrocarbon Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics