You might be wondering what photoelectric effect actually means and why it’s even important. Well, in your photoelectric effect class 11 chapter, this topic pops up early—and for good reason. It explains how electrons are ejected from a metal when light, like ultraviolet, hits it with enough energy.
So, what is photoelectric effect? When light hits certain metals, some of them start kicking out tiny particles—those are electrons. But here’s the thing: not every metal reacts the same. A few only respond if the light has a lot of energy, like ultraviolet light. Others, like sodium or potassium, don’t need that much. Even regular visible light works.
We call those metals photosensitive. Basically, they react when light falls on them. The electrons they spit out? Scientists later called them photoelectrons. And the name for the whole thing, where light knocks electrons loose? That’s the photoelectric effect
While we have given you the basic idea in this introduction, now, we will move on to the detailed sections covering the photoelectric effect definition, then photoelectric effect formula, which helps you calculate the energy involved. There are also a few photoelectric effect laws to understand, like the law of the photoelectric effect, which connects light’s frequency with electron emission. And then you will also come across the Einstein photoelectric effect equation—sometimes written as Einstein's photoelectric equation—which explains everything using the concept of energy quanta.
Photoelectric effect is an important concept covered in Dual nature of Radiation and Matter which is included in class 12th NCERT thus making it important for CBSE board students.
- What is Photoelectric Effect?
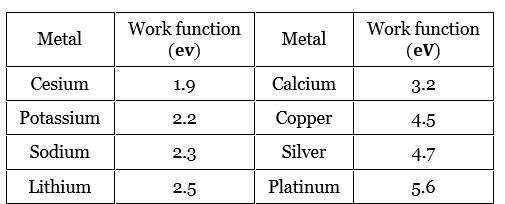
- Work Functions of Some Photosensitive Metals
- Photoelectric Effect Definition as Per NCERT
- Einstein's Photoelectric Effect Equation
- Real-Life Applications of the Photoelectric Effect
What is Photoelectric Effect?
Let us first understand what photoelectric effect is in detail. The phenomenon known as the photoelectric effect occurs when electromagnetic radiation of an appropriate wavelength strikes a metallic surface, causing electrons to be released.
Work Functions of Some Photosensitive Metals
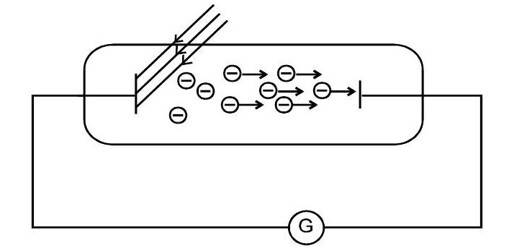
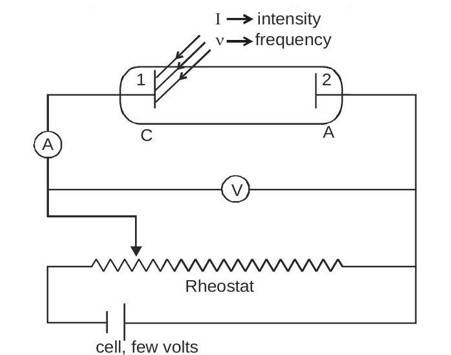
Only metal and light are needed to create the photoelectric effect, but the circuit is finished for viewing. A configuration used to investigate the photoelectric effect is depicted in the figure.
Photoelectric Effect Definition as Per NCERT
“It was found that certain metals like zinc, cadmium, magnesium, etc., responded only to ultraviolet light, having short wavelength, to cause electron emission from the surface. However, some alkali metals such as lithium, sodium, potassium, caesium and rubidium were sensitive even to visible light. All these photosensitive substances emit electrons when they are illuminated by light. After the discovery of electrons, these electrons were termed as photoelectrons. The phenomenon is called photoelectric effect.”
So, when light hits certain metals, some of them start kicking out tiny particles—those are electrons. But here’s the thing: not every metal reacts the same. A few only respond if the light has a lot of energy, like ultraviolet. Others, like sodium or potassium, don’t need that much. Even visible light works.
We call those metals photosensitive substance. Basically, they react when light falls on them. The electrons they spit out? Scientists later called them photoelectrons. And the name for the whole thing, where light knocks electrons loose? That’s the photoelectric effect. Simple, but it ended up being kind of a big deal in physics.
Einstein's Photoelectric Effect Equation
Let us now discuss the Einstein photoelectric effect equation in this section. As per Einstein, light is a wave that interacts with matters as quantum/packet of energy. The photon is quantum of radiation and the equation is known as the Einstein photoelectric effect equation. Einstein derived his equation and subsequently expanded Planck's notion.
According to Einstein, light consists of discrete energy packets called photons. When a photon hits the surface of a metal, it transfers its energy to an electron. If this energy is large enough to overcome the metal’s work function (the minimum energy needed to release an electron), the electron is emitted.
Einstein expressed this with the equation:
As per Einstein's photoelectric effect equation, the arriving photon has an energy of hvh\nuhν.
No matter how strong the light is, no electron is released if the photon's energy is less than the work function.
The notion that light acts as both a wave and a particle (photon) was validated by Einstein's equation. He was awarded the Nobel Prize in Physics in 1921 for this explanation, which also served as the basis for quantum mechanics.
Dual nature of radiation and matter is also an important chapter for JEE Mains exams and NEET exam.
Real-Life Applications of the Photoelectric Effect
The following examples illustrate how the photoelectric effect is used in real-world situations:
- Automatic streetlights use photoelectric or photocell cells. The photoelectric effect is used to detect changes in light intensity in the evening, causing the lights to automatically turn on.
- When sunlight strikes semiconductors in solar panels, electrons are liberated, producing electricity. The Einstein photoelectric effect equation is directly applied to renewable energy in this way.
- To assist photographers gain the proper exposure, light meters in cameras measure the amount of incident light using the photoelectric principle.
- Infrared sensors and TV remote controls both use the photoelectric effect, in which light-based impulses cause particular electronic reactions in the equipment.
It is advisable to practice the NCERT solutions of the chapter to perform well in the examinations.
Physics Dual Nature of Radiation and Matter Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test