- What is Photoelectric Emission?
- Photoelectrons
- Laws of Photoelectric Emission
- Photoelectric Emission in Class 12
- Illustrated Examples
- FAQs
What is Photoelectric Emission?
When a beam of light hits the surface of metals like sodium, potassium, rubidium, and caesium, their electrons start to emit rays. This whole process is known as photoelectric emission.
In simple words, it is a process in which the electrons start to liberate themselves from the metal surface and roam freely in the light. It depends upon the frequency of the light and not the intensity. The frequency of the light and intensity of the light should not be confused with each other.
Photoelectrons
Some metals like alkaline and sodium require less amount of energy to move the electrons from the surface of metals for them to absorb the light. The electrons emitted from this particular type of process is called photoelectrons. The current generated from this same procedure is known as the photoelectric current.
The intensity of light comes into play to determine the number of electrons emitted in the light and the frequency of the radiations. This particular property of the metal can also be used to identify the electrons’ frequency in the light off the surface.
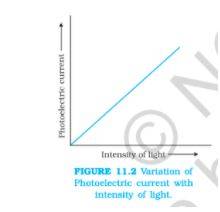
Image source: ncert
The variation of the photoelectric current with light intensity can be understood in detail by the above diagram.
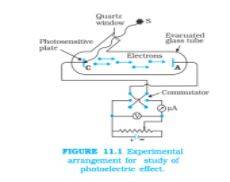
Let’s have a look at the experimental arrangement of the photoelectric effect in detail:
Image source: ncert
This diagram shows the detailed experimental arrangement of the photoelectric effect.
Laws of Photoelectric Emission
Some laws are helpful in the understanding of the photoelectric emissions concept. These laws are as follows:
- The process of emission of electrons from the surface of the metals can only occur if there is enough energy present on the surface of the metal to let the electron emit freely in the light.
- The light on the surface is directly proportional to the number of electrons emitted from the surface in a one-second timeframe.
- The energy possessed in the emitted electrons depends on the frequency of light flux and not on the number of photons.
Photoelectric Emission in Class 12
The chapter ‘Dual Nature of Radiation and Matter’ holds a weightage of 4 marks, and it consists of one short question of three marks and one objective type question of only one mark. ‘Photoelectric Emission’ does not have significant relevance in terms of exams, but it is crucial to make a strong base.
Illustrated Examples
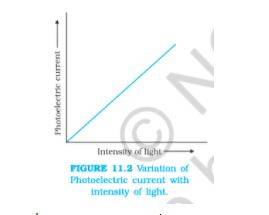
1. Illustrate the relationship between the intensity of light and photoelectric current.
Answer –
Image source: ncert
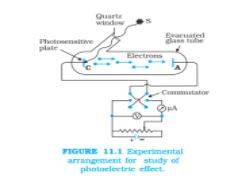
2. Illustrate the experimental state of arrangement for photoelectric emission.
Answer –
Image source: ncert
3. Explain one law of photoelectric emission.
Answer - The energy possessed in the emitted electrons depends on the frequency of light flux and not on the number of photons on the metal surface.
FAQs
Q: What do you mean by photoelectrons?
Q: What role does the intensity of light play?
Q: What is the law of photoelectric emission?
Q: Which gas is present inside photoelectric cells?
Q: During the process, the radiation strikes on which part of the photoelectric cell?
Physics Dual Nature of Radiation and Matter Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test