
Suppose, we shine light onto a piece of metal. If the light has a high enough frequency, tiny particles of it—called photons—transfer their energy to the electrons in the metal. If a photon carries enough energy, it can kick an electron right out of the material.
These freed electrons are known as photoelectrons.
What puzzled scientists for a long time was the fact that only light of a high enough frequency could free electrons, but the intensity of light did not play any role in freeing electrons. This did not fit with what classical physics predicted.
What is Photoelectric Effect?
The photoelectric effect describes what happens when light hits a metal surface and causes electrons to escape from it. This simple idea turned out to be one of the most important discoveries in modern physics, leading directly to the development of quantum theory. In further sections, we will be explaining why photoelectric effect and wave theory of light are out of sync and how quantum theory explains the effect.
Do note that Dual Nature of Radiation and Matter is an important chapter for JEE Mains and NEET exam aspirants.
- Photoelectric Effect and Wave Theory of Light
- Why Wave Theory Cannot Explain Photoelectric Effect?
- Planck's Quantum Theory: Explains Photoelectric Effect
Photoelectric Effect and Wave Theory of Light
As per NCERT:
“The classical wave theory could not explain the main features of photoelectric effect. Its picture of continuous absorption of energy from radiation could not explain the independence of Kmax on intensity, the existence of n o and the instantaneous nature of the process. Einstein explained these features on the basis of photon picture of light. According to this, light is composed of discrete packets of energy called quanta or photons. Each photon carries an energy E (= h n) and momentum p (= h/l), which depend on the frequency (n ) of incident light and not on its intensity. Photoelectric emission from the metal surface occurs due to absorption of a photon by an electron. “
In simple terms:
The classical wave theory is not able to explain the main parts of the photoelectric effect. It said that energy from light is absorbed slowly and continuously. But this could not explain why the maximum energy of electrons (Kmax) does not depend on light’s brightness, why there is a minimum required frequency (called ν0\nu_0ν0), or why electrons are released instantly.
Einstein explained that light is made up of small energy packets that are known as photons. Every photon has that depends on the frequency of light instead of its brightness. Photoelectric emission happens whenever one photon hits an electron and gives it enough energy to escape from the metal.
Please note that students who are currently in class 12 CBSE board must check out the NCERT solutions of Dual Nature of Radiation and Matter.
Why Wave Theory Cannot Explain Photoelectric Effect?
The energy crossing per unit area per unit time perpendicular to the direction of propagation is called the intensity of a wave. Consider a cylindrical volume with area of crosssection A and length c
along the
-axis. The energy contained in this cylinder crosses
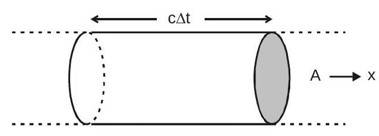
the area A in time as the wave propagates at speed c . The energy contained.
The intensity is
.
In the terms of maximum electric field,
.
If we consider the light as a wave, then the intensity will depend upon electric field.
If we take work function , then
Therefore, for photoelectric effect, there should be a time lag since metal has work function.
However, it has been observed that photoelectric effect is an instantaneous process.
Hence, light is not of wave nature.
- The intensity problem: According to wave theory of light, when the intensity of the light beam grows, so does the amplitude of the oscillating electric field vector E of the light wave. Given that eE is the force acting on the electron, increasing the intensity of the light beam should likewise increase the kinetic energy of the photoelectrons. Observation, however, indicates that the maximal kinetic energy is unaffected by the intensity of the light.
- The frequency problem: The photoelectric effect should happen at any light frequency, according to wave theory of light, as long as the light is strong enough to produce the energy required to expel the photoelectrons. Nevertheless, measurements indicate that each surface has a certain cutoff or threshold frequency, . For frequencies below , the photoelectric effect is absent, regardless of how strong the light beam is.
- The time delay problem: The "effective target area" for the electron in the metal is constrained and most likely not much larger than a circle with a diameter about equal to that of an atom if the energy obtained by a photoelectron is immediately absorbed from the wave incident on the metal plate. The light energy is dispersed evenly over the wavefront in the classical theory. Therefore, there should be a detectable lag between the light's impact on the surface and the photoelectron's ejection if the light is weak enough. The electron should be taking in energy from the beam during this time until it has enough to break free. But no temporal lag that can be detected has been measured till date.
Planck's Quantum Theory: Explains Photoelectric Effect
The light energy from a source will always be an integral multiple of a smaller energy value called quantum of light. Hence energy is
,
where
and
(number of photons)
.
Here energy is quantized.
is known as the quantum of energy, it's a packet of energy called as photon.
While understanding the correlation between photoelectric effect and wave theory of light is important, there are many other important topics in Dual Nature of Radiation and Matter which must be covered as well to have a strong foundation for board and entrance examinations.
Physics Dual Nature of Radiation and Matter Exam
Student Forum
Other Class 12th Physics Chapters
- Physics Alternating Current
- Physics Ray Optics and Optical Instruments
- Physics Electromagnetic Induction
- Physics Dual Nature of Radiation and Matter
- Physics Semiconductor Devices
- Physics Wave Optics
- Physics Current Electricity
- Physics Nuclei
- Physics Electrostatic Potential and Capacitance
- Physics Atoms
- Physics Moving Charges and Magnetism
- NCERT Class 12 Notes
- NCERT Class 12 Physics
- Physics Electric Charge and Field
- Physics Electromagnetic Waves
- Physics Magnetism and Matter
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
