If you are going through your second Physics textbook for Class 11 in CBSE, follow along this guide to learn a bit more on the Measurement of Temperature. We elaborate on this important section of Chapter 10, Thermal Properties of Matter.
By learning the concepts today, you can practice along with our free NCERT Solutions for Class 11 Physics from Shiksha-curated experts. In no time, you will confidently secure three to five marks in your annuals. Plus, you will have a much stronger foundation during your NEET exam preparation.
- What is Temperature Measurement?
- Different Scales of Temperature
- Comparison between Different Temperature Scales
- Thermometers
- Types of Thermometers
- The constant-volume gas thermometer
What is Temperature Measurement?
Temperature measurement is thermometry. It's a method to measure how hot or cold something is. That is possible with the use of special devices that we today know as thermometers.
So, the measurement of temperature becomes an important topic to learn when you want to learn the mechanism of thermometers. That includes a few things
- How they give correct readings
- What scales are there for measuring the temperature, and
- How to convert from one scale to another.
We will discuss all below.
Different Scales of Temperature
When learning about temperature measurement, there are four types of scales.
(a) The Centigrade or Celsius scale
(b) The Fahrenheit scale
(c) The Reaumer scale
(d) Kelvin scale of temperature (K)
Below, we explain how temperature is measured using a general formula.
Comparison between Different Temperature Scales
The formula for the conversion between different temperature scales is:
= = =
General formula for the conversion of temperature from one scale to another:
=
Thermometers
Thermometers are device that are used to measure temperatures. All thermometers are based on the principle that some physical property of a system changes as the system temperature changes.
Required properties of good thermometric substance.
(1) Non-sticky (absence of adhesive force)
(2) Low melting point (in comparison with room temperature)
(3) High boiling temperature
(4) Coefficient of volumetric expansion should be high (to increase accuracy in measurement).
(5) Heat capacity should be low.
(6) Conductivity should be high
Mercury (Hg) suitably exhibits above properties.
Types of Thermometers
When learning about thermometry, it's ideal you learn about the types of thermometers, their properties, why they are good or bad, and the different use cases.
| Type of thermometer and its range |
Thermometric property |
Advantages |
Disadvantages |
Particular Uses |
||
| Mercury-in-glass to |
Length of column of mercury in capillary tube |
(i) Quick and easy to (direct reading) (ii) Easily portable |
(i) Fragile (ii) Small size limits (iii) Limited range |
(i) Every laboratory use where high accuracy is not required. (ii) Can be calibrated against constant-volume gas thermometer for more accurate work |
||
| Constant-volume gas thermometer to |
Pressure of a fixed mass of gas at constant volume |
(i) Very accurate (ii) Very sensitive (iii) Wide range (iv) Easily reproducible |
(i) Very large volume of bulb (ii) Slow to use and inconvenient |
(i) Standard against which others calibrated (ii) or used depending on range (iii) can be corrected to the ideal gas scale (iv) Used as standard below- |
||
| Platinum resistance to |
Electrical resistance of a platinum coil |
(i) Accurate (ii) Wide range |
Not suitable for varying temperature (i.e., is slow to respond to changes) |
|
||
| Thermocouple to |
Emf produced between junctions of dissimilar metals at different temperatures for measurement of emfs |
(i) Fast response because of low heat capacity. (ii) wide range (iii) can be employed for remote readings using long leads. |
Accuracy is lost if emf is measured using a moving-coil voltmeter (as may be necessary for rapid changes when potentiometer is unsuitable) |
(i) Best thermometer for small steady temperature differences (ii) Can be made direct reading by calibrating galvanometer (iii) Used as standard between and |
||
| Radiation pyrometer above |
Colour of radiation emitted by a hot body |
Does not come into contact when temperature is measured |
(i) Cumbersome (ii) Not direct reading (needs a trained observer) |
(i) Only thermometer possible for very high temperatures (ii) Used as standard above . |
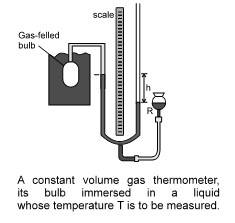
The constant-volume gas thermometer
The standard thermometer, against which all other thermometers are calibrated, is based on the pressure of a gas in a fixed volume. Figure shows such a constant volume gas thermometer; it consists of a gas-filled bulb connected by a tube to a mercury monometer.
Pressure at the temperature being measured, pressure when bulb in a triple point cell.
Physics Thermal Properties of Matter Exam
Student Forum
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion