Specific Heat Capacity is a fundamental concept for understanding the thermal properties of a matter. When a substance comes in contact with heat, it undergoes many changes. The amount of heat absorbed or rejected by a substance to change its temperature is its heat capacity. Specific heat capacity is defined as the amount of heat required to raise the temperature of 1 kilogram (kg) of substance by one degree Celsius, without undergoing phase change. This topic is important for understanding the change of state, heat transfer, calorimetry, and Newton’s law of cooling.
Students can go through the article for detailed understating of special heat capacity topics. A good grip over the topics will help to score good marks in the CBSE exam. Also, students can check NCERT Class 11 Physics solutions for various problems based on specific heat capacity.
- What is Specific Heat Capacity Definition?
- Explanation of Specific Heat Capacity
- Relation between Specific heat and Water equivalent
- Specific Heat Capacity Formula
- Phase change
What is Specific Heat Capacity Definition?
The definition of specific heat capacity, as per the NCERT textbook, is ‘The specific heat capacity is the property of the substance which determines the change in the temperature of the substance (undergoing no phase change) when a given quantity of heat is absorbed (or given off) by it. It is defined as the amount of heat per unit mass absorbed or given off by the substance to change its temperature by one unit.
Also Read: Class 11 Chapter 10 Thermal Properties of Matter NCERT Solution | NCERT Solutions
Explanation: As per the above definition of specific heat capacity, it tells us how much heat is required to change the temperature of a substance.
- If a substance has a high heat capacity, then it requires more heat to change its temperature
- If the heat capacity of a substance is low, then it requires less heat to change the temperature.
Importance of Specific Heat Capacity
Knowledge of specific heat capacity of a substance is important in many industrial, scientific and everyday contexts. Below is its importance.
Importance of Specific Heat Capacity
- Engineering and Material Selection
- Thermal regulation
- Biological system
- Climate and Weather
- Cooking and Food Science
- Space and Aerospace Application
- Industrial process
Explanation of Specific Heat Capacity
Below is the explanation of Specific Heat and Heat Capacity or Thermal Capacity.
Specific Heat
Specific heat of a substance is the amount of heat required to raise or lower its temperature by one unit mass of substance by . When a body is heated, it gains heat and loses it when the body is cooled. The gain or loss of heat is directly proportional to:
(a) The mass of the body
(b) rises or falls of temperature of the body
where is a constant and is known as the specific heat of the body . The S.I. unit of is joule/kg-Kelvin, and the C.G.S. unit is cal./gm .
Specific heat of water:
Specific heat of steam = half of the specific heat of water = the specific heat of ice.
Heat Capacity or Thermal Capacity
The amount of heat required to raise the temperature of that body by is the heat capacity of the substance. If ' ' is the mass and 's' the specific heat of the body, then the Heat capacity . Units of heat capacity in: CGS system are cal ; the S.I. unit is ,
Important Points:
(a) We know, , if the substance undergoes the change of state which occurs at constant temperature , then . Thus, the specific heat of a substance is infinite when it melts or boils at constant temperature.
(b) If the temperature of the substance is changed without any heat transfer, then . Thus, when liquid in the thermos flask is shaken, its temperature increases without the transfer of heat and hence the specific heat of the liquid in the thermos flask is zero.
(c) To raise the temperature of saturated water vapour, heat must be withdrawn. Hence, the specific heat of saturated water vapour is negative. (This is for your information only and not in the course).
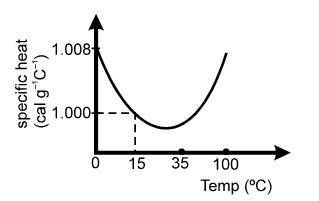
(d) The slight variation of the specific heat of water with temperature is shown in the graph at 1 atmosphere pressure. The variation in specific heat is less than over the interval from 0 to .
Relation between Specific heat and Water equivalent
It is the amount of water which requires the same amount of heat for the same temperature rise as that of the object
is also represented by W
So,
.
Specific Heat Capacity Formula
The formula for specific heat capacity is
Were,
- C is the specific heat capacity (J/kg·°C)
- Q is heat absorbed or released (J)
- ‘m' denotes mass of substance (in kg)
- Change in temperature is denoted by ΔT (in °C or K)
- S.I. unit of specific heat capacity is J kg-1 K-1.
Phase change
Heat required for the change of phase or state is, latent heat.
Latent heat (L): The heat supplied to a substance which changes its state at constant temperature is
called the latent heat of the body.
Latent heat of Fusion : The heat supplied to a substance which changes it from a solid to a liquid state at
its melting point and 1 atm. pressure is called the latent heat of fusion. The latent heat of fusion of ice is 80
kcal/kg
Latent heat of vaporisation : The heat supplied to a substance which changes it from a liquid to a vapour
state at its boiling point and 1 atm. pressure is called the latent heat of vaporisation. The latent heat of vaporisation of water is .
Latent heat of ice:
Latent heat of steam: L =
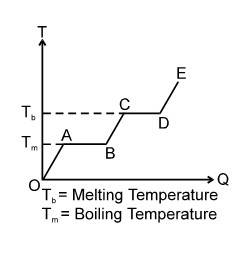
The given figure represents the change of state by different lines
where the mass ( ) of substance constant slope of the graph, is inversely proportional to specific heat, if in the given diagram
(slope) (slope) DE
then
when
If (length of ) (length of )
then (latent heat of AB) > (latent heat of CD)
Physics Thermal Properties of Matter Exam
Student Forum
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion