The change of state is a process in action that tells us when a substance is changing from a solid to liquid to gas or in any other order. This is a phase transition where we consider a solid to be one phase, the liquid (or gas) to be another. The way physics sees this change happening is how energy emits or absorbs among the transitions that occur. One of the important topics in the Thermal Properties of Matter chapter Class 11, change of state has an important use in exams that you must follow.
- What is Change of State?
- Latent Heat and Change of State
- Phase Transition Temperature Conditions
- Heating Curve with Change of State
- Principle of Calorimetry for Change of State
- NCERT Revision Notes Physics Class 11
- NCERT Solutions for Physics Class 11
What is Change of State?
Change of state is a transformation process of a substance from one physical state to another where energy absorbs or releases. The only consideration for this process is the temperature has to be constant which scientifically is well-known as the isothermal process. At the same time, the change from one state, such as from liquid to solid, sees a shift of potential energy in the substance iself. Now that will happen even when the temperature does not change.
Latent Heat and Change of State
The condition that we were earlier stressing on, that the temperature is not changing but there is a change in energy. Now that's the energy of heat, well- or formerly-known as the latent heat, which leads to change of state.
Let's see the physics behind that.
We need to know the heat that a mass absorbs or releases.
For a mass we have the quantity Q that appears in your thermodynamics chapter. Q tells us how heat energy gets transferred in and out of the system as there is some temperature difference.
See this equation here, to understand.
where is the latent heat and the measurements in the SI unit format is or .
There are two more concepts that aid to the concept of latent heat.
- Latent Heat of Fusion : All we should be covering is that it's the heat that is generally required to convert 1 kg of solid to liquid. That can happen at its melting point.
For water at , we will have the Latent Heat of Fusion as
- Latent Heat of Vapourisation : The heat that we would need to change 1 kg of liquid to gas when it's at the boiling point.
For water at , we have the Latent Heat of Vapourisation as
Just know that the latent heat of vaporisation will naturally be above the latent of fusion. Now this occurs, because the volume increases a lot more when there is a change of state from liquid phase to gas. The energy to come out of the pull for intermolecular forces is more here, as compared to transitioning from solid to liquid.
Phase Transition Temperature Conditions
As we now know that the change of state happens when there is no temperature change, there's more to it. Not every physical state will change naturally. Instead there is another factor, where the physical state of the substance has to be at particular temperatures. There are three to cover here.
- Melting Point: The shift from solid to liquid states occur only at the melting point. What is happening here is that the heat absorbed increases potential energy. In the chemical form, that creates a breaking of lattice bonds.
- Boiling Point: The liquid to gas change happens at the boiling point. Heat can push away the attractive forces at the intermolecular level. That leads to the expansion of volume.
- Solidification and Condensation: Reverse processes release latent heat.
We just to remember that all these transitions are isothermal in nature, which tells us that temperature will remain constant until the phase change gets completed.
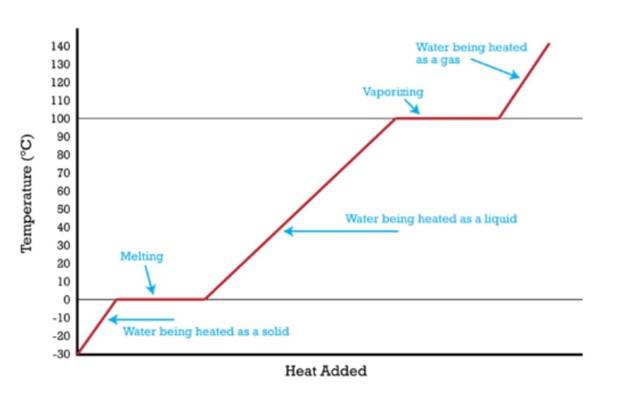
Heating Curve with Change of State
Below, we will show the heating curve in terms of temperature versus the heat that's supplied.
- Solid Phase: Temperature rises linearly. This happens as heat leads to an increase in kinetic energy ( ).
- Melting: A plateau at the melting point, where heat equals the latent heat of fusion .
- Liquid Phase: Temperature rises again .
- Boiling: A plateau at the boiling point, absorbing the latent heat of vaporisation .
- Gas Phase: Temperature increases further.
The slope of temperature-time curves is inversely proportional to specific heat, and plateau lengths reflect latent heat magnitudes.
Heating Curve for Water at 1.00 atm Pressure
Principle of Calorimetry for Change of State
In Class 11 Physics, we need to approach most of the numericals for change of state using the simple Principle of Calorimetry.
It follows the basic principles of physics, such as the energy conservation concept for two bodies in a system, where total energy is preserved between even when one body loses energy, the other body will gain it. The Principle of Calorimetry is the same, where if a hotter body loses energy, it's temperature will be equal to the heat gained by a cooler body.
If we consider a mixture of masses , and they remain at two different temperatures . Now they will have specific heats as represented by :
The equilibrium temperature for that situation would be appearing as
NCERT Revision Notes Physics Class 11
Explore the notes below.
| Units and Measurements Class 11 Notes | Mechanical Properties of Solids Class 11 Notes |
| Motion in a Straight Line Class 11 Notes | Mechanical Properties of Fluids Class 11 Notes |
| NCERT Class 11 Notes for Motion in a Plane | Thermal Properties of Matter Class 11 Notes |
| Laws of Motion Class 11 Notes | Thermodynamics Class 11 Notes |
| Work, Energy, and Power Class 11 Notes | Kinetic Theory of Gas Class 11 Notes |
| System of Particles and Rotational Motion Class 11 Notes | Oscillations Class 11 Notes |
| Gravitation Class 11 Notes | Waves Class 11 Notes |
| NCERT Class 11 Notes for PCM |
| NCERT Class 11 Physics Notes |
NCERT Solutions for Physics Class 11
Check for NCERT Solutions for Physics Class 11. Or look below for each chapter.
Commonly asked questions
When phase changes happen, why do the plateaus of temperature show?
You will be seeing these temperature plateaus appearing on the heating curve at the main melting and boiling points. The reason for that is the energy reaches latent heat. And we know that latent heat is the energy that lets phase transitions occur without increasing the temperature.
How is latent heat related to change of state?
It's the energy that is needed for a change of state to transition from one substance or physical state to another. Remember that the main condition for latent heat is that there is no temperature change when the energy is absorbed or released.
Physics Thermal Properties of Matter Exam
Student Forum
Other Class 11th Physics Chapters
- Physics Mechanical Properties of Solids
- NCERT Class 11 Physics
- NCERT Class 11 Notes
- NCERT Notes
- Physics Motion in Plane
- Physics Mechanical Properties of Fluids
- Physics Motion in Straight Line
- Physics System of Particles and Rotational Motion
- Physics Oscillations
- Physics Waves
- Physics Thermal Properties of Matter
- Physics Motion
- Physics Gravitation
- Physics Thermodynamics
- Physics Work, Energy and Power
- Physics Units and Measurement
- Physics Laws of Motion