
NCERT Solution Class 11 Environmental Chemistry: Candidates are provided here the NCERT solutions for Class 11 Environmental Chemistry chapter 14. The subject expert at Shiksha has prepared the Environmental Chemistry NCERT class 11 solutions to help students perform well in the exam. NCERT Class 11 Solutions pdf is prepared focusing on the exam difficulty level, previous year questions, and competitive exam standards. Students who prepare for their board or competitive exam, such as JEE, NEET, using the Environmental Chemistry class 11 NCERT solutions can clear the test with good scores. However, it is important to devote plenty of time to the preparation. Moreover, it is important to prepare the Chapter 14 class 11 chemistry environmental chemistry notes for quick revision and recalling all chemical properties, reactions, atomic numbers and more.
In competitive exams like JEE Main and NEET, at least one question is asked every year from this chapter. Also, in the annual exams of the school, this chapter has its share of questions. It is thus advised to study the NCERT class 11 Chemistry Environmental Chemistry chapter thoroughly and score good marks in the exam.
The Environmental Chemistry chapter Class 11 NCERT deals with various types of pollution and the measures to control them. Waste management is one important topic covered in this chapter and about which every student must be aware due to the rising pollution of various kinds, which include atmospheric pollution, water pollution, soil pollution, industrial pollution, among others. Students will also learn how green belt management will help us protect the environment. Students preparing for entrance exams can refer NCERT Solutions Chemistry class 12.
* NCERT Class 11 Chemistry Chapter 14 Environmental Chemistry is removed from the CBSE Class 11 Chemistry syllabus.
- NCERT Class 11 Chemistry Chapter 14 - Topics
- Environmental Chemistry Solutions Latest
- Environmental Chemistry Solutions More Solution
- Benefit of using NCERT Solutions for Class 11 Chemistry Chapter 14
- NCERT Chemistry Chapter 14 Environmental Chemistry
NCERT Class 11 Chemistry Chapter 14 - Topics
Below is the list of topics covered in the NCERT Class 11 Chemistry Chapter 14, Environmental Chemistry.
| Topics | Subtopics |
| Environmental Pollution |
- |
| Atmospheric Pollution |
|
| Water Pollution |
|
| Soil Pollution |
|
| Industrial Waste |
- |
| Strategies to Control Environmental Pollution |
|
| Green Chemistry |
|
Environmental Chemistry Solutions Latest
Here we have provided shorts answer type questions based on Class 11 Environmental Chemistry. These types of questions carry 2 marks.
| 14.1. Define environmental chemistry? |
| Answer: Environmental chemistry is a branch of environmental studies. Environmental studies deal with the sum of all social, economical, biological, physical and chemical interrelations with our surroundings. Environmental chemistry deals with the study of the origin, transport, reactions, effects and fates of chemical species in the environment |
| 14.2. Explain the tropospheric pollution in 100 words? |
| Answer: Tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The following are the major gaseous and particulate pollutants present in the troposphere: 1. Gaseous air pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants. 2. Particulate pollutants: These are dust, mist, fumes, smoke, smog etc. |
| 14.3. Carbon monoxide gas is more dangerous than carbon dioxide gas. Why? |
| Answer: Carbon monoxide binds to haemoglobin to form carboxyhaemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. In blood, when the concentration of carboxyhaemoglobin reaches about 3–4 per cent, the oxygen-carrying capacity of blood is greatly reduced. This oxygen deficiency results into headache, weak eyesight, nervousness and cardiovascular disorder. This is the reason why people are advised not to smoke. In pregnant women who have the habit of smoking the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies. On the other hand, CO2 does not combine with haemoglobin and hence is less harmful than CO. |
| 14.4. List gases which are responsible for greenhouse effect. |
| Answer: CO2 is mainly responsible for the greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapours, CFCs (chlorofluorocarbons) and Ozone. |
Commonly asked questions
14.1. Define environmental chemistry?
Environmental chemistry is a branch of environmental studies. Environmental studies deal with the sum of all social, economical, biological, physical and chemical interrelations with our surroundings. Environmental chemistry deals with the study of the origin, transport, reactions, effects and fates of chemical species in the environment
14.2. Explain the tropospheric pollution in 100 words?
Tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The following are the major gaseous and particulate pollutants present in the troposphere:
1. Gaseous air pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
2. Particulate pollutants: These are dust, mist, fumes, smoke, smog etc.
14.3. Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Carbon monoxide binds to haemoglobin to form carboxyhaemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. In blood, when the concentration of carboxyhaemoglobin reaches about 3–4 per cent, the oxygen-carrying capacity of blood is greatly reduced. This oxygen deficiency results into headache, weak eyesight, nervousness and cardiovascular disorder. This is the reason why people are advised not to smoke. In pregnant women who have the habit of smoking the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies. On the other hand, CO2 does not combine with haemoglobin and hence is less harmful than CO.
14.4. List gases which are responsible for greenhouse effect.
CO2 is mainly responsible for the greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapours, CFCs (chlorofluorocarbons) and Ozone.
14.5. Statues and monuments in India are affected by acid rain. How?
This is mainly due to the large number of industries and power plants in the nearby areas. Acid rain has vapours of sulphuric acid dissolved in it. When it comes in contact with various statues or monuments, the acid reacts chemically with marble (calcium carbonate).
CaCO3 + H2SO4 à CaSO4 + H2O + CO2
As a result, the monument is being slowly disfigured, and the marble is getting discoloured and lustreless.
14.6. What is smog? How is classical smog different from photochemical smogs?
The word smog is a combination of smoke and fog. It is a type of air pollution that occurs in many cities throughout the world. Classical smog occurs in a cool, humid climate. It is also called reducing smog. Whereas photochemical smog (photo means light) occurs in warm and dry sunny climates. It has a high concentration of oxidising agents and therefore, it is also called oxidising smog.
14.7. Write down the reactions involved during the formation of photochemical smog.
Mechanism of formation of photochemical smog:
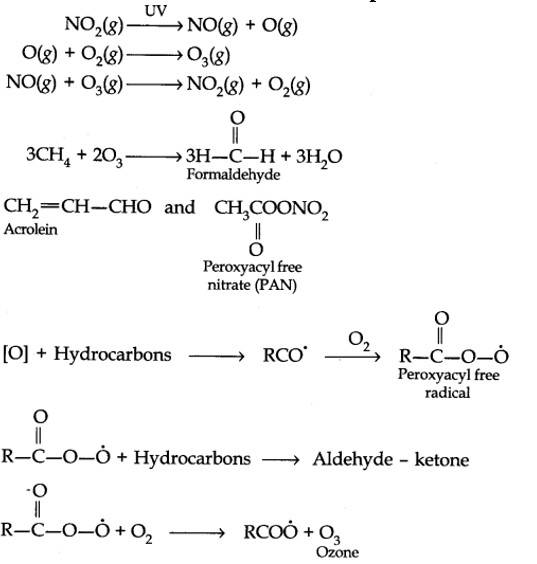
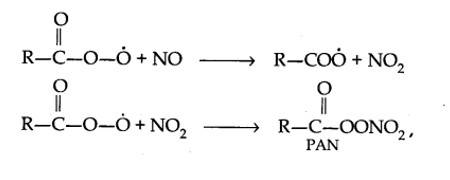
14.8. What are the harmful effects of photochemical smog and how can they be controlled?
Harmful effects of photochemical smog: Photochemical smog causes serious health problems. Both ozone and PAN (peroxyacetyl nitrate) act as powerful eye irritants. Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing. Photochemical smog leads to cracking of rubber and extensive damage to plant life. It also causes corrosion of metals, stones, building materials, rubber and painted surfaces.
Control:
- Control of primary precursors of photochemical smog, such as NO2 and hydrocarbons, the secondary precursors such as ozone and PAN, the photochemical smog will automatically be reduced.
- Use of catalytic converter in automobiles prevents the release of nitrogen oxide and hydrocarbons to the atmosphere.
Plants like Pinus, juniparus, quercus, pyrus etc. can metabolise nitrogen oxide thus their plantation could help in this matter.
14.10. What do you mean by ozone hole? What are its consequences?
Depletion of ozone layer creates some sort of holes in the blanket of ozone which surrounds us, this is known as ozone hole.
The consequences are:
- With the depletion of ozone layer, more UV radiation filters into troposphere. UV radiations lead to ageing of skin, cataract, sunburn, skin cancer, killing of many phytoplanktons, damage to fish productivity etc.
- It has also been reported that plant proteins get easily affected by UV radiations which leads to the harmful mutation of cells.
- It also increases evaporation of surface water through the stomata of the leaves and decreases the moisture content of the soil.
4. Increase in UV radiations damage paints and fibres, causing them to fade faster.
14.11. What are the major causes of water pollution? Explain.
Major Causes of water pollution are:
Pathogens: Pathogens include bacteria and other microorganisms that enter water from domestic sewage and animal excreta.
Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis, which cause gastrointestinal diseases.
Organic wastes: Organic wastes, when added to water, are decomposed by bacteria, which consume dissolved oxygen in water. When the concentration of dissolved oxygen in water decreases below 6 ppm, the growth of fish is inhibited. The breakdown of organic wastes by anaerobic bacteria produces chemicals that have a foul smell and are harmful to human health.
Chemical pollutants: Some inorganic chemicals as an industrial wastes dissolve in water like cadmium, mercury nickel etc. These metals are dangerous to humans and other animals. These metals can damage kidneys and central nervous system, liver etc. Petroleum products pollute many sources of water.
14.12. Have you ever observed any water pollution in your area? What measures would you suggest to control it?
Yes, we observed water pollution near our area due to various human activities like toxic discharges from factories and industrial plants, runoff from agricultural fields, domestic wastes etc.
It can be controlled by preventing the toxic chemicals from entering the water bodies. Regular checks on contamination or rivers, lakes or ponds of toxic compounds need to be done. Use of chemical fertilizers should be avoided to prevent the harmful chemicals from entering the ground water.
14.13. What do you mean by Biochemical Oxygen Demand (BOD)?
The amount of oxygen required by bacteria to breakdown the organic matter present in a certain volume of a sample of water is called Biochemical Oxygen Demand (BOD).
14.14. Do you observe any soil pollution in your neighbourhood? What efforts will you make for controlling the soil pollution?
The major sources of soil pollution are industrial wastes and agricultural pollutants like fertilizers and pesticides. Insecticides like DDT are insoluble in water and are absorbed by plant roots. Many pesticides like Aldrin and Dieldrin are toxic and non-biodegradable, which can enter the higher trophic levels through food chains, causing metabolic and physiological disorders. Industrial wastes comprise of several toxic metals such as Pb, As, Hg, Cd etc.
To control soil pollution, the addition of pollutants to the soil should be avoided. Waste materials should undergo proper treatment and recycling before dumping.
14.15. What are pesticides and herbicides? Explain giving examples.
Pesticides are the chemical compounds used to control the damage caused by pests (insects, rodents etc.)
Example: Aldrin, Dieldrin, B.H.C etc.
Herbicides are the chemicals used to control weeds.
Example: Triazines, Sodium chlorate (NaClO3), sodium arsenite (Na3AsO3).
14.16. What do you mean by green chemistry? How will it help in decrease environmental pollution?
Green chemistry is a way of thinking and is about utilising the existing knowledge and principles of chemistry and other sciences to reduce the adverse effect of pollution. It is a production process that would bring about minimum pollution or deterioration to the environment. In a nutshell, it is a cost-effective approach which involves reduction in material, energy consumption and waste generation.
It will help in decreasing environmental pollution by adopting the following examples:
- In a chemical reaction, if reactants are fully converted into useful environmental friendly products by using an environment friendly medium then there would be no chemical pollutants introduced in the environment.
- During a synthesis, care must be taken to choose starting materials that can be converted into end products with yield approximately up to 100 per cent.
- It may be worthwhile to carry out synthetic reactions in aqueous medium since water has high specific heat and low volatility. Water is cost effective, non inflammable and devoid of any carcinogenic effects.
14.17. What would have happened if the greenhouse gases were totally missing in the Earth’s atmosphere? Discuss.
The solar energy radiated back from the Earth's surface is absorbed by the greenhouse gases (CO2, CH4, O3, CFCs) present near the Earth's surface.
They heat up the atmosphere near the Earth's surface and keep it warm. As a result of these, there is the growth of vegetation which supports life. In the absence of this effect, there would be no life for both plants and animals on the surface of the Earth.
14.18. A large number of fish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill.
Excessive phytoplankton (organic pollutants such as leaves, grass trash etc.) present in water are biodegradable. Bacteria decompose these organic matters in water. During this process, when a large number of bacteria decompose these organic matters, they consume the dissolved oxygen in water. When the level of dissolved oxygen falls below 6 ppm the fish cannot survive.
14.19. How can domestic waste be used as manure?
Domestic waste can be separated into biodegradable and non-biodegradable materials. Non-biodegradable materials such as plastic, glass, metal scraps etc. are sent for recycling. Biodegradable wastes can be deposited in landfills and are converted into compost to be used as manure.
14.20. For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odour, flies and recycling of wastes for a good produce.
The compost producing pit should be kept covered so that flies cannot make entry into it and bad odour is minimized.
The waste materials which are non-biodegradable like glasses, plastic bags, plastic bottles, polybags, must be handed over to the vendors who can send them to the recycling plants.
14.22 Assertion: CO is a colourless and odourless gas, highly poisonous to living beings.
Reason: It is produced as a result of incomplete combustion of carbon and is mainly released into the air by automobile exhaust.
(b) CO is highly poisonous to living beings because of its ability to block the delivery of oxygen to the organs and tissues.
14.3 Assertion: Tetra chlroroethene was earlier used as solvent for dry cleaning, which now a days are discontinued.
Reason: Chlorine gas was used earlier for bleaching.
(b) The compound contaminates the groundwater and is also a suspected carcinogen.
MCQs
14.26 Which of the following statements is incorrect?
(a) The atmosphere that surrounds the earth isnot of the same thickness at all heights.
(b) The lowest regionof atmosphere in which the human beingsalong with other organisms live is calledtroposphere.
(c) Below the troposphere,between 10 and 50 km above sea level lies stratosphere.
(d) Troposphere is a turbulent, dusty zone containing air, much water vapour and clouds.
(c) The Stratosphere is above the troposphere.
14.2 The main constituents of air are
(a) N2 and O2 (b) SO2 and CO2 (c) CO and CO2 (d) none of the above
(a) N2 and O2
14.28 The pollutant responsible for Bhopal gas tragedy was
(a) Ammonia (b) Methyl isocyanate (c) Nitrous oxide (d) Mustard gas
(b) Methyl isocyanate
14.29 Read the following statements carefully. Select the incorrect statement.
(a) Besides carbon dioxide, other greenhouse gases are methane, water vapour, nitrous oxide, CFCs and ozone.
(b) Methane is produced naturally when vegetation is burnt, digested or rotted in the absence of oxygen.
(c) Large amounts of methane are released in paddy fields, coal mines, from rotting garbage dumps and by fossil fuels.
(d) Methane are man-made industrial chemicals used in air conditioning etc.
(d) Chlorofluorocarbons (CFCs) are man-made industrial chemicals used in air conditioning etc.
14.5. Photochemical smog occurs in
(a) Warm, dry and sunny climate (b) cool (c) humid (d) none of the above.
(a) warm, dry and sunny climate.
14.34. What is the full form of PCBs and what are they?
PCBs are polychlorinated biphenyls. They are contaminants of water. They are used as fluids in transformers and capacitors.
14.35. Cite examples where green chemistry can be applied.
Green chemistry can be applied as mentioned in the following examples:
(i) In dry-cleaning, the use of liquefied CO2 in place of tetrachloroethene.
(ii) In the bleaching of paper using hydrogen peroxide instead of chlorine.
(iii) In the manufacture of chemicals like ethanol, using environment-friendly chemicals and conditions.
14.7. List out the gases which are considered as major source of air pollution.
Carbon monoxide (CO), sulphur dioxide (SO2) and oxides of nitrogen (NO2).
14.38. What are insecticides? Give examples of insecticides.
A Toxic substance that is used to kill insects is called an insecticide. For example: DDT, BHC.
14.39. What should be the tolerable limit of F ions in drinking water?
1 ppm or 1 mg dm-3.
14.40. Define (a) eutrophication (b) pneumoconiosis (c) Photochemical smog (d) Classical smog
(a) Eutrophication: When the growth of algae increases in the surface of water, dissolved oxygen in water is reduced. This phenomenon is known as eutrophication. (Due to this growth of fish gets inhibited).
(b) Pneumoconiosis: It is a disease which irritates lungs. It causes scarring or fibrosis of the lung.
(c) Photochemical smog: Photochemical smog is formed as a result of the photochemical decomposition of nitrogen dioxide and chemical reactions involving hydrocarbons. It takes place during dry warm season in the presence of sunlight. It is oxidising in nature.
(d) Classical smog: Classical smog is formed due to the condensation of SO, vapours on particles of carbon in a cold climate. It is generally formed during winter when there is severe cold. It is reducing in nature.
14.9. What are the reactions involved for ozone layer depletion in the stratosphere?
Kindly go through the solution
14.1 Assertion: Atmosphere trapping the sun’s heat near the earth’s surface is called natural greenhouse effect.
Reason: Atmosphere trapping the sun’s heat maintains the temperature and makes the earth perfect for life.
Kindly go through the solution
(a)
14.4 Assertion: As insect resistance of DDT increased, other organic toxins such as Aldrin and Dieldrin were introduced in the market by pesticide industry.
Reason: The repeated use of the same or similar pesticides give rise to pests that are resistant to that group of pesticides thus making the pesticides ineffective.
Kindly go through the solution
(a)
14.5 Assertion: It is important to maintain the ozone shield ozone (O3).
Reason: O3 protects us from the harmful ultraviolet (UV) radiations coming from the sun.
Kindly go through the solution
(a)
14.1. Which acid is most abundant in acid rain?
Kindly go through the solution
H2SO4
14.2. BOD is a measure of
Organic pollutants in water
14.3. Ozone depletion is mainly due to
Kindly go through the solution
CFCs
Environmental Chemistry Solutions More Solution
A few more questions on Environmental Chemistry Class 11 NCERT are provided below. These are questions and answers based on the Class 11 Environmental Chemistry NCERT book solutions.
| 14.5. Statues and monuments in India are affected by acid rain. How? |
| Answer: This is mainly due to the large number of industries and power plants in the nearby areas. Acid rain has vapours of sulphuric acid dissolved in it. When it comes in contact with various statues or monuments, the acid reacts chemically with marble (calcium carbonate). |
| 14.6. What is smog? How is classical smog different from photochemical smogs? |
| Answer: The word smog is a combination of smoke and fog. It is a type of air pollution that occurs in many cities throughout the world. Classical smog occurs in a cool, humid climate. It is also called reducing smog. Whereas photochemical smog (photo means light) occurs in warm and dry sunny climates. It has a high concentration of oxidising agents and therefore, it is also called oxidising smog. |
| 14.7. Write down the reactions involved during the formation of photochemical smog. |
| Answer: Mechanism of formation of photochemical smog: |
| 14.8. What are the harmful effects of photochemical smog and how can they be controlled? |
| Answer: Harmful effects of photochemical smog: Photochemical smog causes serious health problems. Both ozone and PAN (peroxyacetyl nitrate) act as powerful eye irritants. Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing. Photochemical smog leads to cracking of rubber and extensive damage to plant life. It also causes corrosion of metals, stones, building materials, rubber and painted surfaces. Control:
|
| 14.10. What do you mean by ozone hole? What are its consequences? |
| Answer: Depletion of ozone layer creates some sort of holes in the blanket of ozone which surrounds us, this is known as ozone hole. The consequences are:
|
| 14.11. What are the major causes of water pollution? Explain. |
| Answer: Major Causes of water pollution are: Pathogens: Pathogens include bacteria and other microorganisms that enter water from domestic sewage and animal excreta. Organic wastes: Organic wastes, when added to water, are decomposed by bacteria, which consume dissolved oxygen in water. When the concentration of dissolved oxygen in water decreases below 6 ppm, the growth of fish is inhibited. The breakdown of organic wastes by anaerobic bacteria produces chemicals that have a foul smell and are harmful to human health. Chemical pollutants: Some inorganic chemicals as an industrial wastes dissolve in water like cadmium, mercury nickel etc. These metals are dangerous to humans and other animals. These metals can damage kidneys and central nervous system, liver etc. Petroleum products pollute many sources of water. |
| 14.12. Have you ever observed any water pollution in your area? What measures would you suggest to control it? |
| Answer: Yes, we observed water pollution near our area due to various human activities like toxic discharges from factories and industrial plants, runoff from agricultural fields, domestic wastes etc. It can be controlled by preventing the toxic chemicals from entering the water bodies. Regular checks on contamination or rivers, lakes or ponds of toxic compounds need to be done. Use of chemical fertilizers should be avoided to prevent the harmful chemicals from entering the ground water. |
| 14.13. What do you mean by Biochemical Oxygen Demand (BOD)? |
| Answer: The amount of oxygen required by bacteria to breakdown the organic matter present in a certain volume of a sample of water is called Biochemical Oxygen Demand (BOD). |
| 14.14. Do you observe any soil pollution in your neighbourhood? What efforts will you make for controlling the soil pollution? |
| Answer: The major sources of soil pollution are industrial wastes and agricultural pollutants like fertilizers and pesticides. Insecticides like DDT are insoluble in water and are absorbed by plant roots. Many pesticides like Aldrin and Dieldrin are toxic and non-biodegradable, which can enter the higher trophic levels through food chains, causing metabolic and physiological disorders. Industrial wastes comprise of several toxic metals such as Pb, As, Hg, Cd etc. To control soil pollution, the addition of pollutants to the soil should be avoided. Waste materials should undergo proper treatment and recycling before dumping. |
| 14.15. What are pesticides and herbicides? Explain giving examples. |
| Answer: Pesticides are the chemical compounds used to control the damage caused by pests (insects, rodents etc.) |
| 14.16. What do you mean by green chemistry? How will it help in decrease environmental pollution? |
| Answer: Green chemistry is a way of thinking and is about utilising the existing knowledge and principles of chemistry and other sciences to reduce the adverse effect of pollution. It is a production process that would bring about minimum pollution or deterioration to the environment. In a nutshell, it is a cost-effective approach which involves reduction in material, energy consumption and waste generation.
|
| 14.17. What would have happened if the greenhouse gases were totally missing in the Earth’s atmosphere? Discuss. |
| Answer: The solar energy radiated back from the Earth's surface is absorbed by the greenhouse gases (CO2, CH4, O3, CFCs) present near the Earth’s surface. |
| 14.18. A large number of fish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill. |
| Answer: Excessive phytoplankton (organic pollutants such as leaves, grass trash etc.) present in water are biodegradable. Bacteria decompose these organic matters in water. During this process, when a large number of bacteria decompose these organic matters, they consume the dissolved oxygen in water. When the level of dissolved oxygen falls below 6 ppm the fish cannot survive. |
| 14.19. How can domestic waste be used as manure? |
| Answer: Domestic waste can be separated into biodegradable and non-biodegradable materials. Non-biodegradable materials such as plastic, glass, metal scraps etc. are sent for recycling. Biodegradable wastes can be deposited in landfills and are converted into compost to be used as manure. |
| 14.20. For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odour, flies and recycling of wastes for a good produce. |
| Answer: The compost producing pit should be kept covered so that flies cannot make entry into it and bad odour is minimized. The waste materials which are non-biodegradable like glasses, plastic bags, plastic bottles, polybags, must be handed over to the vendors who can send them to the recycling plants. |
Benefit of using NCERT Solutions for Class 11 Chemistry Chapter 14
The benefits of using the NCERT Class 11 Environmental Chemistry solutions are mentioned below.
- Clear Concept Explanation: The NCERT explains the complex topics in Environmental Chemistry in easy-to-understand language, which makes the conceptual foundation strong.
- Accurate and Reliable: NCERT Class 11 Chemistry solutions are based on the textbook and follow the CBSE exam pattern. Students can use the Environmental Chemistry Class 11 questions and answers to perform well in the board exams.
- Effective Revision Tool: The questions and answers help in quick revision, recalling concepts and reactions. Regular practice helps to make a good grip over topics, formulas, and reactions.
- Improve answer writing skills: The answers in the NCERT Class 11 Chemistry Chapter 14 Environmental Chemistry are mentioned systematically, which will help students to understand the correct way to answer a question.
NCERT Chemistry Chapter 14 Environmental Chemistry
Students can check the Environmental Chemistry FAQs below.
Commonly asked questions
With the help of subject experts at Shiksha we have provided the NCERT solutions for environmental chmistry class 11 with explanation through this article.
Yes, Class 11 Chemistry Environmental Chemistry notes are benefical in quick revision. Environmental Chemistry notes must consist of detailed explanation of topics, formulas, diagrams and reactions.
NCERT Environmental Chemistry short questions and answers are provided in this artciles. These types of questions help in boosting the marks.
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
