
Class 11 Chemistry Ch 1 NCERT solutions explore some basic concepts of chemistry. Modern Chemistry is not a very old science discipline. However, its traces can be seen in ancient India and also in China. The class 11 chemistry chapter 1 begins with the history of Chemistry in various ancient Indian books. Later, it covers basic concepts such as the characteristics of three states of matter, substance classification into elements, mixtures, and compounds, SI base units, and physical quantities conversion from one system of units to another.
The NCERT solutions provide some basic concepts of chemistry, including the significance of molecular mass, atomic mass, average atomic mass, and formula mass. It explains various laws of chemical combination. It describes the mole, molar mass, empirical formula, and molecular formula for compounds, and more. Students can also download the free Some Basic Concepts of Chemistry Class 11 PDF and get well-structured NCERT Solutions.
The class 11 science students can find the comprehensive NCERT solutions for Maths, Physics, and Chemistry here. They will get chapter-wise key topics and free PDFs.
- Class 11 Some Basic Concepts of Chemistry: Key Topics, and Weightage
- Important Formulas of Some Basic Concepts of Chemistry Class 11
- Class 11 Chemistry Some Basic Concepts of Chemistry NCERT Solution PDF: Download PDF for Free
- Some Basic Concepts of Chemistry Questions and Answers
- Some Basic Concepts - FAQs
Class 11 Some Basic Concepts of Chemistry: Key Topics, and Weightage
Class 11 Some Basic Concepts of Chemistry is an important chapter from a competitive exam point of view. A few important topics for the NEET exam include stoichiometry, mole concept, and chemical equations, and for the JEE Main exam, other than these topics, the following are also important: the empirical, molar mass, percentage composition, and molecular formulas.
It is always a good idea to know in advance the topics covered in a chapter. The following are the topics covered in Chapter 1, Some Basic Concepts of Chemistry:
| Exercise | Topics Covered |
|---|---|
| 1.1 | Importance of Chemistry |
| 1.2 | Nature of Matter |
| 1.3 | Properties of Matter and Their Measurement |
| 1.4 | Uncertainty in Measurement |
| 1.5 | Laws of Chemical Combinations |
| 1.6 | Dalton's Atomic Theory |
| 1.7 | Atomic and Molecular Masses |
| 1.8 | Mole Concept and Molar Masses |
| 1.9 | Percentage Composition |
| 1.10 | Stoichiometry and Stoichiometric Calculations |
Some Basic Concepts of Chemistry Weightage in NEET, JEE Main Exams
| Exam | Weightage |
|---|---|
| NEET | 6-8% |
| JEE Main | 8-9% |
Important Formulas of Some Basic Concepts of Chemistry Class 11
Important Concepts and Formulae of Class 11 Some Basic Concepts of Chemistry
Mole Concept and Molar Mass
- Atomic Mass Unit (amu): 1 amu = of the mass of one carbon-12 atom.
- Mole: A quantity containing entities (Avogadro’s number).
- Number of Moles (n):
- Number of Atoms/Molecules:
where
Empirical and Molecular Formula
- Relation between Molecular and Empirical Formula:
Stoichiometry and Stoichiometric Calculations
- Percentage Composition:
- Mass-Volume Relationship (at STP):
Concentration Terms in Solutions
- Mass Percent (w/w%):
- Molarity (M):
- Molality (m):
- Normality (N):
- Mole Fraction (χ):
Class 11 Chemistry Some Basic Concepts of Chemistry NCERT Solution PDF: Download PDF for Free
Shiksha has compiled the complete Class 11 Chemistry Some Basic Concepts Solutions for the NCERT Textbook in one place. This Class 11 Chemistry Chapter 1 NCERT solution PDF is a very useful study material for school exams and competitive exams such as NEET, JEE Mains, SAAT, and others. Students can access the Chapter 1 NCERT solution PDF from the link given below;
Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry NCERT Solution PDF: Download Free PDF
Some Basic Concepts of Chemistry Questions and Answers
| 1.1. Calculate the molecular mass of the following: |
| Answer: (i) Molecular mass of H2O = (2x Atomic mass of Hydrogen)+ Atomic mass of Oxygen Atomic mass of Hydrogen = 1.008 amu Atomic mass of Oxygen = 16.00 amu So, Molecular mass of H2O = 2(1.008 amu) + 16.00 amu =18.016 amu
Atomic mass of Carbon = 12 amu
Atomic mass of Oxygen = 16 amu So, Molecular mass of CO2 = 12.01 amu + 2 x 16.00 amu = 44.01 amu
Atomic mass of Carbon = 12 amu Atomic mass of Hydrogen = 1.008 amu So, Molecular mass of CH4 = 12.01 amu + 4 (1.008 amu) = 16.042 amu |
| 1.2. Calculate the mass percent of different elements present in sodium sulphate (Na2SO4). |
| Answer: Molar mass of Na2SO4= (2 x Atomic mass of Sodium) + Atomic mass of Sulphur + (4 x Atomic mass of Oxygen) = (2x 23) + 32 + (4x 16) = 46 + 32 + 64 = 142 g/mol |
| 1.3. Determine the empirical formula of an oxide of iron, which has 69.9% iron and 30.1 % dioxygen by mass. |
| Answer: We are given that the percentage of iron by mass is 69.9% and the percentage of oxygen by mass is 30.1%. Since we have relative moles of both elements, we can calculate the simpler molar ratio of iron to oxygen = 1.25: 1.88 Divide by the smaller value to both = 1.25/1.25: 1.88/1.25 = 1: 1.5 = 2: 3 So, now we can write the empirical formula of iron oxide as Fe2O3. |
| 1.4. Calculate the amount of carbon dioxide that could be produced when (i) 1 mole of carbon is burnt in air. (ii) 1 mole of carbon is burnt in 16 g of dioxygen. (iii) 2 moles of carbon are burnt in 16 g of dioxygen. |
| Answer: In order to answer the question, we need to know the balanced equation for the combustion of carbon in dioxygen/air, which can be written as: C (s) + O2 (g) à CO2 (g) (i) We can see from the above equation, 1 mole of carbon reacts with 1 mole of oxygen to produce 1 mol of carbon dioxide. In air, combustion is complete. Therefore, CO2 produced from combustion of 1 mole of carbon= Molar mass of CO2= 44 g (ii) As only 16 g of dioxygen is available, it can combine only with 0.5 mole of carbon, i.e., dioxygen is the limiting reactant. Hence, CO2 produced = 22 g Here, dioxygen acts as the limiting reagent. (iii) Here again, dioxygen is the limiting reactant. 16 g of dioxygen can combine only with 0.5 mole of carbon (even though 2 moles of carbon are available). Therefore, the CO2 produced again is equal to 22 g. |
Commonly asked questions
1.24. Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation:
N2 (g) + 3H2(g) —–> 2NH3 (g)
(i) Calculate the mass of ammonia produced if 2.00 × 103 g dinitrogen reacts with 1.00 ×103 g of dihydrogen.
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
1.24. According to the given equation, 1 mol of N2 reacts with 3 mol of H2.
Or, 28 g of N2 react with 6 g of H2.
So, 2000 g of N2 will react with H2 = 6/28 x 2000 g = 428.6 g of H2.
(i) 2 mol of N2 or 28 g of N2 produce NH3 = 2 mol = 34 g
So, 2000 g of N2 will produce NH3 = 34/28 x 2000 g = 2428.57 g
(ii) Yes, N2 is the limiting reagent while H2 is the excess reagent. So, H2 will remain unreacted.
(iii) H2 will remain unreacted. Mass left unreacted = 1000 g – 428.6 g = 571.4 g
1.5. Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol-1.
1.5
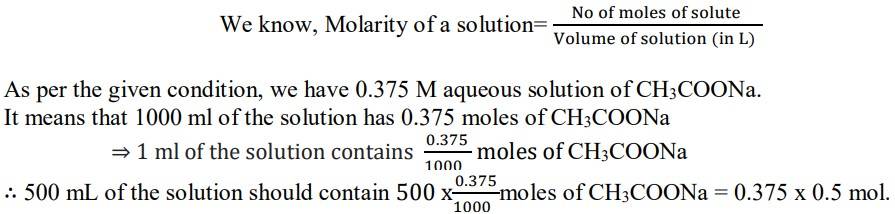
Since the molar mass of sodium acetate is 82.0245 g mol-1.
⇒ 1 mol of CH3COONa has a mass = 82.0245 g
∴Mass of sodium acetate (CH3COONa) required to make 500 ml of 0.375 molar aqueous solution
= 0.375 x 0.5 mol x 82.0245 g mol-1
= 15.3795 g = 15.380 g
1.21. The following data were obtained when dinitrogen and dioxygen react together to form compounds: (Beginner)
Mass of dinitrogen Mass of dioxygen
(i) 14 g 16 g
(ii) 14 g 32 g
(iii) 28 g 32 g
(iv) 28 g 80 g
(a) Which law of chemical combination is obeyed by the above experimental data? Give its statement.
(b) Fill in the blanks in the following conversions:
(i) 1 km = ...................... mm = ...................... pm
(ii) 1 mg = ...................... kg = ...................... ng
(iii)1 mL = ...................... L = ...................... dm3
1.21. (a) Fixing the mass of dinitrogen as 28 g, masses of dioxygen combined will be 32,64, 32 and 80 g in the given four oxides. Theseare in the ratio 1: 2: 1: 5 which is a simple whole number ratio. Hence, the given data obeys the law of multiple proportions.
(b)
(i) 1 km = 103 m and 1 m = 103 mm. So, 1 km = 103 x 103 mm = 106 mm
Now, 1 pm = 10-12 m. So, 1 km = 103 m x 1012 m = 1015 pm
Therefore, 1 km = 106 mm = 1015 pm
(ii) 1 mg = 10-3 g and 1 g = 10-3kg. So, 1 mg = 10-3 x 10-3 kg = 10-6 kg
Now, 1 mg = 10-3 g and 1 g =
Therefore, 1 mg = 10-6 kg = 106 ng
1L = 1000 mL.So 1 mL = 10-3 L.
Now, 1 mL = 1cm3 and 1dm = 10cm. So, 1 mL = 10-3 dm3
Therefore, 1 mL = 10-3 L = 10-3 dm3
1.10. In three moles of ethane (C2H6), calculate the following:
(i) Number of moles of carbon atoms
(ii) Number of moles of hydrogen atoms
(iii) Number of molecules of ethane
1.10. (i) 1 mole of C2H6 contains 2 moles of carbon atoms
Therefore, no of moles of C atoms in 3 moles of C2H6 = 6 moles
(ii) 1 mole of C2H6 contains 6 moles of hydrogen atoms
Therefore, no of moles of H atoms in 3 moles of C2H6 = 18 moles
(iii) 1 mole of C2H6 contains 6.02 x 1023 molecules
Therefore, 3 moles of C2H6 will contain ethane molecules = 3 x 6.02 x 1023 molecules= 18.06 x 1023 molecules
1.6. Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL-1 and the mass percent of nitric acid in it is being 69%.
1.6. A mass percent of 69% means that 100 g of nitric acid solution contains 69 g of nitric acid by mass.
Molar mass of nitric acid HNO3= 1 + 14 + (3x16) = 63 gmol-1
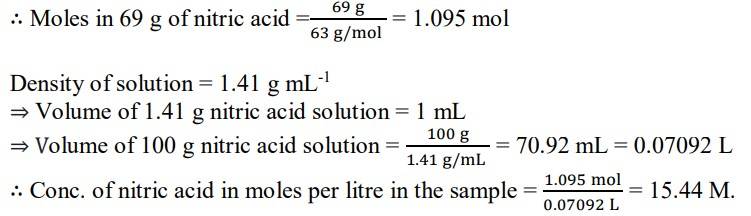
1.9. Calculate the atomic mass (average) of chlorine using the following data:
|
|
% Natural Abundance |
Molar Mass |
|
35Cl |
75.77 |
34.9689 |
|
37Cl |
24.23 |
36.9659 |
1.9. Average atomic mass = (Fractional abundance of 35Cl x molar mass of 35Cl) + (Fractional abundance of 37Cl x molar mass of 37Cl)
= (75.77/100 x 34.9689) + (24.23/100 x 36.9659)
= 26.4959 + 8.9568
= 35.4527
1.23. In the reaction,
A + B2——> AB2,
identify the limiting reagent, if any, in the following reaction mixtures
(i) 300 atoms of A + 200 molecules ofB
(ii) 2 mol A + 3 mol B
(iii) 100 atoms of A + 100 molecules of B
(iv) 5 mol A + 2.5 mol B
(v) 2.5 mol A + 5 mol B
1.23. (i) According to the given reaction, 1 atom of A reacts with 1 molecule of B
Thus, 200 molecules of B will react with 200 atoms of A and 100 atoms of A will be left unreacted. Hence, B is the limiting reagent while A is the excess reagent.
(ii) According to the given reaction, 1 mol of A reacts with 1 mol of B
Thus, 2 mol of A will react with 2 mol of B. Hence, A is the limiting reactant since 1 mol of B is left unreacted.
(iii) No limiting reagent.
(iv) 2.5 mol of B will react with 2.5 mol of A. Hence, B is the limiting reagent.
(v) 2.5 mol of A will react with 2.5 mol of B. Hence, A is the limiting reagent
1.20. Round up the following upto three significant figures:
(i) 34.216
(ii) 10.4107
(iii) 0.04597
(iv) 2808
1.20. The values after round up with three significant figures are:
(i) 34.2
(ii) 10.4
(iii) 0.0460
(iv) 2810
1.12. If the density of methanol is 0.793 kg L -1, what is its volume needed for making 2.5 L of its 0.25 M solution?
1.12. Density of methanol = 0.793 kg/L, molar mass of methanol (CH3OH) = 32g/mol = 0.032 kg/mol
V1 =? , V2 = 2.5 L, M2 = 0.25 M
We can apply the formula of
M1V1 = M2V2
Or V1 = M2V2/M1
Substituting M1 = density / molar mass, we get
M1 = 0.793/0.032 = 24.78
V1 = 0.25 x 2.5 / 24.78 = 0.02522 L = 25.22 mL
1.31. How many significant figures should be present in the answer of the following calculations?
(i) 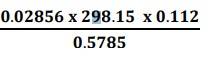
(ii) 5 × 5.364
(iii) 0.0125 + 0.7864 + 0.0215
1.31. First we need to find the least precise number to find the significant figures.
(i) Least precise number of the calculation![]() is 0.112
is 0.112
Therefore, number of significant figures in the answer = Number of significant figures in the least precise number, i.e. 3
(ii) Least precise number of calculations = 5.364
Therefore, number of significant figures in the answer will be = Number of significant figures in 5.364 = 4
(iii) 0.0125+0.7864+0.0215
Since the least number of decimal places in each term is four, the number of significant figures in the answer will also be 4.
1.34. A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0 L (measured at S.T.P.) of this welding gas is found to weigh 11.6 g. Calculate (i) empirical formula, (ii) molar mass of the gas, and (iii) molecular formula.
1.34. (i) 1 mole (44 g) of CO2 will have 12 g carbon.
So, 3.38 g of CO2 will have carbon = 12g/44g * 3.38
= 0.9217 g
18 g of water will have 2 g of hydrogen.
So, 0.690 g of water contain hydrogen = 2g/18g * 0.6902g
= 0.0767 g
Since carbon and hydrogen are the only constituents of the compound, the total mass of the compound is:
= 0.9217 g + 0.0767 g
=0.9984 g
So, the percentage of Carbon in the compound = 0.9217/0.9984 * 100 = 92.32%
Now, percentage of Hydrogen in the compound = 0.0767/0.9984 * 100 = 7.68%
Moles of carbon in the compound = 92.32/12=7.69
Moles of hydrogen in the compound = 7.68/1=7.68
Since, we have the number of moles of both the elements, the ratio of carbon to hydrogen will be:
7.69: 7.68 = 1: 1
This is in the whole number, so the empirical formula will be CH.
(ii) Molar mass of the gas
Ans: We are given,
Weight of 10.0 L of the gas (at S.T.P) = 11.6 g
Since, 1mol of a gas occupies 22.4L volume at STP. So,
Weight of 22.4L of gas at STP = 11.6g/10.0L * 22.4L
=25.984g
≈26g
Hence, the molar mass of the gas is 26 g/mol.
(iii) Molecular formula
Empirical formula mass is CH = 12 + 1= 13 g
No of moles, n = Molar mass of gas/ Empirical formula mass of gas
= 26/13 = 2
Therefore, molecular formula = 2 x CH = C2H2
1.7. How much copper can be obtained from 100 g of copper sulphate (CuSO4)? (Atomic mass of Cu= 63.5 amu).
1.7. 1 mole of CuSO4 contains 1 mole (1 g atom) of Cu
Molar mass of CuSO4= 63.5 + 32 + (4 x 16) = 159.5 g mol-1
Thus, Cu that can be obtained from 159.5 g of CuSO4 = 63.5 g
∴ Cu that can be obtained from 100 g of CuSO4 =63.5/159.5 * 100 = 39.81g
1.13. Pressure is determined as force per unit area of the surface. The S.I. unit of pressure, pascal, is as shown below:
1 Pa = 1 Nm-2.
If mass of air at sea level is 1034 g cm-2, calculate the pressure in pascal.
1.13. Pressure= Force/Area
But weight = m x g, where m = mass (in kg) and g = 9.8 m/s2
Therefore, Pressure = 1034 g cm-2 x 9.8 m/s2
= 1034 x 10-3 kg x (100 m)2 x 9.8 m/s2
= 101332 Pa
= 1.01332 x 105 Pa
1.35. Calcium carbonate reacts with aqueous HCl according to the reaction
CaCO3 (s) + 2HCl (aq) ———->CaCl2 (aq) +CO2(g) +H2O(l).
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
1.35. Step 1: 0.75 M HCl means 0.75 mol per 1000 mL or 0.75 x 36.5 g in 1000 mL.
i.e. 1000 mL of 0.75 M HCl contains 0.75 x 36.5 g HCl
Therefore, 25 mL of 0.75 M HCl contains 0.75 x 36.5 x 25/ 1000 g = 0.6844 g
Step 2: To calculate mass of CaCO3reacting completely with 0.6844 g of HCl
CaCO3 (s) + 2HC1 (aq)———>CaCl2 (aq) +CO2 (g) + H2O
2 mol of HCl, i.e., 2 x 36.5 g = 73 g HCl react completely with CaC03 = 1 mol = 100 g
Therefore, 0.6844 g HCl will react completely with CaCO3 = 100/73 x 0.6844 g = 0.938 g
1.29. Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
1.29. According to the formula of mole fraction,
X (C2H5OH) = 0.040 = n (ethanol) / [n (ethanol) + n (water)]
Now, n (water) = 1000g/18g mol-1 = 55.55 moles [? Density of water=1kg m-3]
Therefore, 0.040 = n (ethanol) / [n (ethanol) + 55.55]
i.e., 0.04 x n (ethanol) + 2.222 = n (ethanol)
i.e., 2.222 = [1- 0.04] n (ethanol)
i.e., n (ethanol) = 2.222 / 0.96 = 2.314 M
1.19. How many significant figures are present in the following?
(i) 0.0025
(ii) 208
(iii) 5005
(iv) 126,000
(v) 500.0
(vi) 2.0034
1.19. The significant figures in the given values are:
(i) 2 in 0.0025
(ii) 3 in 208
(iii) 4 in 5005
(iv) 3 in 126,000
(v) 4 in 500.0
(vi) 5 in 2.0034
1.18. Express the following in scientific notation:
(i) 0.0048
(ii) 234,000
(iii) 8008
(iv) 500.0
(v) 6.0012
1.18. The scientific notation of the given values are:
(i) 4.8*10?3
(ii) 2.34*105
(iii) 8.008*103
(iv) 5.000*102
(v) 6.0012*100
1.28. Which one of the following will have largest number of atoms?
(i) 1 g Au (s) (ii) 1 g Na (s) (iii) 1 g Li (s) (iv) 1 g of Cl2(g) (Atomic masses: Au = 197, Na = 23, Li = 7, Cl = 35.5 amu)
1.28. The number of atoms in each of the given elements are calculated as below:
(i) 1 g Au = 1 / 197 mol = 1/ 197 x 6.02 x 1023 atoms
(ii) 1 g Na = 1/ 23 mol = 1/ 23 x 6.02 x 1023 atoms
(iii) 1 g Li = 1/ 7 mol = 1 / 7 x 6.02 x 1023 atoms
(iv) 1 g of Cl2 = 1/ 71 mol = 1/ 71 x 6.02 x 1023 atoms
Therefore, since the denominator is the smallest in case of Li, 1 g Li has the largest number of atoms
1.46. The concentration of a solution or the amount of substance present in its given volume can be expressed in
(a) Mass per cent or weight percent (w/w %) and mole fraction
(b) Molarity and molality
(c) Both a and b
(d) Neither a nor b
1.46. (c) both a and b
1.17. A sample of drinking water was found to be severely contaminated with chloroform, CHCl3 supposed to be carcinogenic in nature. The level of contamination was 15 ppm (by mass).
(i) Express this in percent by mass
(ii) Determine the molality of chloroform in the water sample.
1.17. 15 ppm means 15 parts per million i.e.15 in 106
So, % by mass = 15/106 x 100 = 15 x 10-4 = 1.5 x 10-3%
Molality = No. of moles of solute/Mass of solvent in kg
Percent by mass = 1.5 x 10-3 % means 100 g of the sample contain 1.5 x 10-3 g chloroform.
So, 1000 g or 1 kg of the sample will contain 1.5 x 10-3 x 1000/100 = 1.5 x 10-2 g chloroform.
Molar mass of chloroform = 12 + 1 + (3 x 35.5) = 119.5 g/mol
Therefore, molality = 1.5 x 10-2 /119.5 = 1.26 x 10-4m.
1.33. Calculate the number of atoms in each of the following:
(i) 52 moles of He
(ii) 52 u of He
(iii) 52 g of He
1.33. (i) 52 moles of Ar
Ans:1 mole of Ar = 6.022 * 1023 atoms of Ar
Therefore, 52 mole of Ar = 52 * 6.022 * 1023 atoms of Ar= 3.131 * 1025 atoms of Ar
(ii) 52 u of He
Ans:1 atom of He = 4u of the He
Or, 4 u of He = 1 atom of He
So, 52 u of He = 52/4 atom of He = 13 atoms of He.
(iii) 52 g of He
Ans:4g of He = 6.022 * 1023 atoms of He
So, 52g of He = 6.022 * 1023 * 52/4 atoms of He
= 7.8286 * 1024 atoms of He
1.11. What is the concentration of sugar (C12H22O11) in mol L -1 if its 20 g are dissolved in enough water to make a final volume up to 2 L?
1.11. Molar mass of sugar = (12 x 12) + (22 x 1) + (11 x 16) =342 g/mol
No. of moles in 352 g of sugar = 1 mol
No. of moles in 20 g = 20 x 1/352 = 0.0585 mol
Therefore, molar concentration = moles of solute / volume of solution in L = 0.0585 / 2 = 0.0293 mol/L
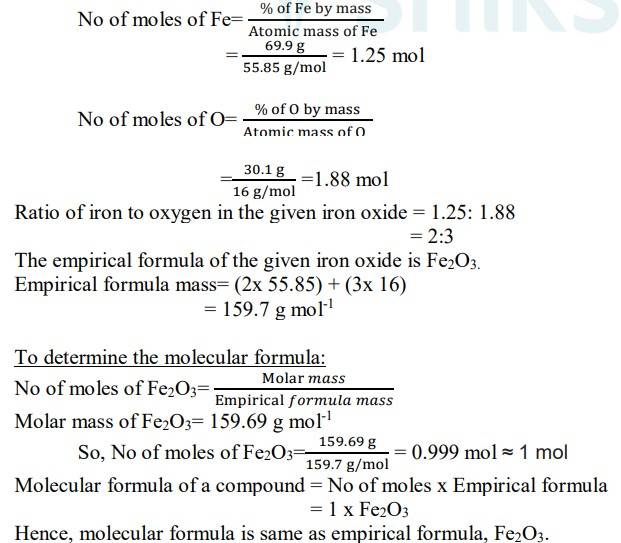
1.8. Determine the molecular formula of an oxide of iron in which the mass percent of iron and oxygen are 69.9 and 30.1 respectively.
1.8. Mass percentage of Fe = 69.9%
Mass percentage of O = 30.1%
1.36. Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction.
4 HCl (aq) + MnO2 (s) ———–> 2 H2O (l) + MnCl2(aq) +Cl2(g)
How many grams of HCl react with 5.0 g of manganese dioxide? (Atomic mass of Mn = 55 u
1.36. The molar mass of MnO2is 87g and the molar mass of HCl is 36.5 g.
According to the equation, 4 moles of HCl (i.e. 4 x 36.5g = 146 g) reacts with 1 mol of MnO2 (87g).
So, for 5.0 g of MnO2 will react with:
= 5 x146/87 = 8.40 g of MnO2
Therefore, 8.4 g of HCl will react with 5 g of MnO2.
1.16. What do you mean by significant figures?
1.16. Significant figures are meaningful digits which are known with certainty plus one which is estimated or uncertain. The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures
1.43. The mass of one atom of C-12 in grams is:
(a) 6.022 × 1023 g
(b) 6.022 × 1023 kg
(c) 1.99 × 10–23 g
(d) 1.99 × 10–23 kg
1.43. (c) The mass of 1 mole of C-12 atoms = 12 g
1 mole of C- atoms = 6.022 * 1023 atoms
The mass of 1 atom of C-12 = 12 / (6.022 * 1023)
= 1.99 * 10–23 g
1.41. Assertion: In chemical reactions, the reactant, which gets consumed first, limits the amount of product formed and is called the limiting reagent.
R: Stoichiometry deals with the calculation of masses (sometimes volumes also) of the reactants and the products involved in a chemical reaction.
1.41. (b) Both the statements are correct but the second statement is not the actual reason for the first statement.
Many a times, reactions are carried out with the amounts of reactants that are different than the amounts as required by a balanced chemical reaction. In such situations, one reactant is in more amount than the amount required by balanced chemical reaction.
The reactant which is present in the least amount gets consumed after sometime and after that further reaction does not take place whatever be the amount of the other reactant. Hence, the reactant, which gets consumed first, limits the amount of product formed and is, therefore, called the limiting reagent
1.32. Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
|
Isotope |
Isotopic molar mass |
Abundance |
|
36Ar |
35.96755g mol-1 |
0.337% |
|
38Ar |
37.96272 g mol-1 |
0.063% |
|
40Ar |
39.9624 g mol-1 |
99.600% |
1.32. Molar mass of argon is
= [ (35.96755 * 0.337/100)+ (37.96272 * 0.063/100)+ (39.9624 * 99.60/100)]g mol-l
= [0.121+0.024+39.802] g mol-l
= 39.947 g mol-l
So, the molar mass of argon is 39.947 g/ mol.
Assertion Type Questions:
1.37. Assertion: One mole of any gas contains 6.022 x 1023 molecules.
Reason: 1 g of any gas contains equal number of moles.
1.37. (c) Equal moles of different substances contain same number of constituent particles but equal weights of different substances do not contain the same number of constituent particles due to the difference in their molar mass.
1.1. Calculate the molecular mass of the following:
(i) H2O (ii) CO2 (iii) CH4
1.1. (i) Molecular mass of H2O = (2x Atomic mass of Hydrogen)+ Atomic mass of Oxygen
Atomic mass of Hydrogen = 1.008 amu
Atomic mass of Oxygen = 16.00 amu
So,
Molecular mass of H2O = 2 (1.008 amu) + 16.00 amu =18.016 amu
(ii) Molecular mass of CO2 = Atomic mass of Carbon + (2x Atomic mass of Oxygen)
Atomic mass of Carbon = 12 amu
Atomic mass of Oxygen = 16 amu
So,
Molecular mass of CO2 = 12.01 amu + 2 x 16.00 amu = 44.01 amu
(iii) Molecular mass of CH4 = Atomic mass of Carbon+ (4x Atomic mass of Hydrogen)
Atomic mass of Carbon = 12 amu
Atomic mass of Hydrogen = 1.008 amu
So,
Molecular mass of CH4 = 12.01 amu + 4 (1.008 amu) = 16.042 amu
1.14. What is the S.I. unit of mass? How is it defined?
1.14. S.I. unit of mass is kilogram (kg).
It is defined as the mass of platinum-iridium block stored at international bureau of weights and measures in France.
1.26. If 10 volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many volumes of water vapour could be produced?
1.26. H2 and O2 react according to the equation
2H2 (g) + O2 (g) ——>2H2O (g)
Thus, 2 volumes of H2 react with 1 volume of O2 to produce 2 volumes of water vapour. Hence, 10 volumes of H2 will react completely with 5 volumes of O2 to produce 10 volumes of water vapour.
1.40. Assertion: SI unit of volume is L (litre).
R: But again, in chemistry laboratories, volumes is often denoted in cm3 or dm3 units.
1.40. (d) Volume is the amount of space occupied by a substance. It has the units of (length)3. In SI system, volume has units of m3. Smaller volumes are denoted in cm3 or dm3 units. A common unit, litre (L) which is not an SI unit, is used for measurement of volume of liquids.
1.38. Assertion: 123.00 has three significant figures.
Reason: All numbers right to the decimal point are significant.
1.38 (d) 123.00 has five significant figures. Assertion is wrong statement while reason is a correct statement.
1.3. Determine the empirical formula of an oxide of iron, which has 69.9% iron and 30.1 % dioxygen by mass.
1.3 We are given that the percentage of iron by mass is 69.9% and the percentage of oxygen by mass is 30.1%.
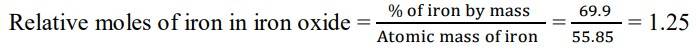
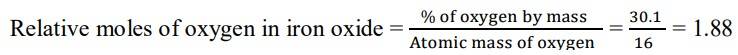
Since we have relative moles of both elements, we can calculate the simpler molar ratio of iron to oxygen
= 1.25: 1.88
Divide by the smaller value to both
= 1.25/1.25: 1.88/1.25
= 1: 1.5
= 2: 3
So, now we can write the empirical formula of iron oxide as Fe2O3.
1.27. Convert the following into basic units:
(i) 28.7 pm
(ii) 15.15 µs
(iii) 25365 mg
1.27. (i) 28.7 pm = 28.7 x 10-12 m = 2.87 x 10-11 m
(ii) 15.15 µs = 15.15 x 10-6 s = 1.515 x 10-5 s
(iii) 25365 mg = 25365 mg x 10-6 kg = 2.5365 x 10-2 kg
1.15. Match the following prefixes with their multiples:
|
Prefixes |
Multiples |
|
(i) micro |
106 |
|
(ii) deca |
109 |
|
(iii) mega |
10–6 |
|
(iv) giga |
10–15 |
|
(v) femto |
10 |
1.15. micro = 10–6, deca = 10, mega = 106, giga = 109, femto = 10–15
1.22. If the speed of light is 3.0 × 108ms–1, calculate the distance covered by light in 2.00 ns.
1.22. Speed = Distance / Time
Or, Distance = Speed x Time = 3.0 * 108ms–1 x 2.00 ns = 3.0 * 108ms–1 x 2.00 x 10-9 s = 6 x 10-1 m = 0.600 m
1.50. One atomic mass unit is defined as one-twelfth of the mass of one carbon-12 atom. Why?
1.50. Carbon-12 isotope is the most abundant isotope of carbon and has been chosen as the standard. Since C-12 is used as the standard atom, one atomic mass unit is defined as one-twelfth of the mass of one carbon – 12 atoms. This is because it has an equal number of protons and neutrons (6) and makes up the majority of matter.
1.51. Write down the proposals of Dalton’s atomic theory.
1.51. In 1808, Dalton published 'A New System of Chemical Philosophy', in which he proposed the following:
1. Matter consists of indivisible atoms.
2. All atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reactions involve reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
1.49. What is the empirical mass of ethene?
1.49. The empirical mass of ethene is half of its molecular mass. The empirical formula represents the simplest whole-number ratio of various atoms present in a compound.
Molecular Formula = n * Empirical formula
Empirical formula of Ethene = C2H4
Empirical Formula Mass = 14 amu= ½ Molecular Mass of Ethene
The ratio of Carbon and Hydrogen in the empirical formula is 1: 2.
1.44. SI unit of Luminous intensity is measured in
(a) Ampere
(b) Candela
(c) flux
(d) lumin
1.44. (b) Candela
1.4. Calculate the amount of carbon dioxide that could be produced when
(i) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16 g of dioxygen.
(iii) 2 moles of carbon are burnt in 16 g of dioxygen.
1.4 In order to answer the question, we need to know the balanced equation for the combustion of carbon in dioxygen/air, which can be written as:
C (s) + O2 (g) à CO2 (g)
(i) We can see from the above equation,
1 mole of carbon reacts with 1 mole of oxygen to produce 1 mol of carbon dioxide.
In air, combustion is complete.
Therefore, CO2 produced from combustion of 1 mole of carbon= Molar mass of CO2= 44 g
(ii) As only 16 g of dioxygen is available, it can combine only with 0.5 mole of carbon, i.e., dioxygen is the limiting reactant.
Hence, CO2 produced = 22 g
Here, dioxygen acts as the limiting reagent.
(iii) Here again, dioxygen is the limiting reactant.
16 g of dioxygen can combine only with 0.5 mole of carbon (even though 2 moles of carbon are available). Therefore, the CO2 produced again is equal to 22 g.
MCQs
1.42. Which of the following statements is correct?
(a) 100 has only one significant figure
(b) 100. has three significant figures
(c) 100.0 has four significant figures
(d) all the above
1.42. (d) all the above
1.45. 16 g of methane on complete combustion gives
(a) 18g of water
(b) 54g of water
(c) 9 g of water
(d) 36 g of water.
1.45. (d) 36 g of water
CH4 + 2O2 → CO2 + 2H2O
1 mol of CH4 reacts with 1 mol O2 of to produce 2 moles of H2O
It means that 16g of CH4 reacts with oxygen to produce (2x18)= 36g of water
1.39. Assertion: Volume of a gas is inversely proportional to the number of moles of gas.
Reason: The ratio by volume of gaseous reactants and products agrees with their mole ratio.
1.39. (d) Taking reference of the ideal gas equation PV= nRT (Where, P= Pressure, V= volume, n=no of moles, R= gas const, T= temperature). It can thus be concluded that volume is directly proportional to the no. of moles.
Question Answers:
1.47. Write down the rules for determining the number of significant figures.
1.47. These rules for determining the number of significant figures are stated below:
(1) All non-zero digits are significant. For example, in 285 cm, there are three significant figures and in 0.25 mL, there are two significant figures.
(2) Zeros preceding to first non-zero digit are not significant. Such zero indicates the position of decimal point. Thus, 0.03 has one significant figure and 0.0052 has two significant figures.
(3) Zeros between two non-zero digits are significant. Thus, 2.005 has four significant figures.
(4) Zeros at the end or right of a number are significant, provided they are on the right side of the decimal point. For example, 0.200 g has three significant figures. But, if otherwise, the terminal zeros are not significant if there is no decimal point.
1.30. What will be the mass of one 12C atom in g?
1.30. 1 mol of 12C atoms = 6.02 x 1023 atoms = 12 g
Or, 6.02 x 1023 atoms of 12C have mass = 12 g
Therefore, 1 atom of 12C will have mass = 12 x 1/6.02 x 1023 = 1.9927 x 10-23 g
1.25. How are 0.50 mol Na2CO3 and 0.50 M Na2CO3 different?
1.25. Molar mass of Na2CO3= (2 x 23) + 12 + (3 x 16) = 106g mol-1
0.50 mol Na2CO3 means 0.50 x 106 g = 53 g of Na2CO3
0.50 M Na2CO3 means 0.50 mol per volume in litre,
i.e., half of 106 g Na2CO3 is present in 1 L solution.
i.e.,53 g Na2CO3 is present in 1 L of the solution
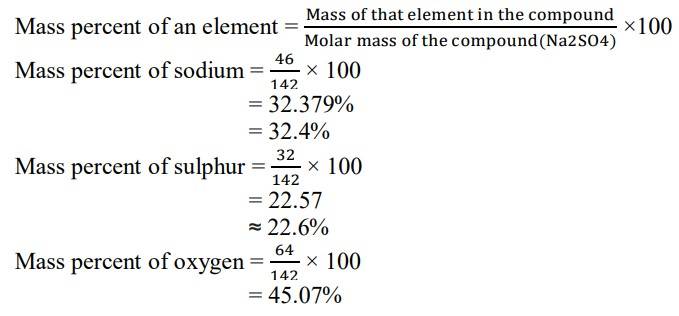
1.2. Calculate the mass percent of different elements present in sodium sulphate (Na2SO4).
1.2 Molar mass of Na2SO4= (2 x Atomic mass of Sodium) + Atomic mass of Sulphur + (4 x Atomic mass of Oxygen)
= (2x 23) + 32 + (4x 16)
= 46 + 32 + 64
= 142 g/mol
1.48. What is the difference between molality and molarity?
1.48.
Molarity | Molality |
The molarity of a given solution is defined as the total number of moles of solute per litre of solution. | Molality is defined as the total moles of a solute contained in a kilogram of a solvent. |
The mathematical expression is- M = number of moles of the solute /Volume of solution given in terms of litres. M = (g ? 1000)/(W ? V). | The mathematical expression is- m = Numbers of moles of solute/Mass of solvent in kgs m = (g ? 1000)/(W ? m). |
Depends on the volume of the whole solution. | Depends on the mass of the solvent. |
Unit sign expressed as (M). | Unit sign expressed as (m). |
Molarity has a unit of mol/litre. | Molality has units of mol/kg. |
Some Basic Concepts - FAQs
Here are the FAQs of this chapter:
Commonly asked questions
What is some basic concepts of chemistry Chapter 1 all about?
As the name suggests, the first chapter of the NCERT Class 11 Chemistry introduces various basic concepts of chemistry, such as the definition and importance of chemistry, atomic matter and molecular masses, the mole concept, laws of chemical combination, empirical, stoichiometry, and molecular formulas. It also includes the concepts of molarity and molality.
Which are the main concepts covered in some basic concepts of chemistry?
The following are the key concepts of this chapter: Compound, Elements, Rules, Law of conservation of mass, Addition and Subtraction, Atomic Mass, Law of multiple proportions, and Molecular Mass.
What is the weightage of some basic concepts of chemistry in NEET exam?
In the medical entrance test NEET, there can be 1 to 3 questions from this chapter. Some year, the Chemistry section of NEET has only one question from this chapter and in some other years, there can be 3 questions.
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
