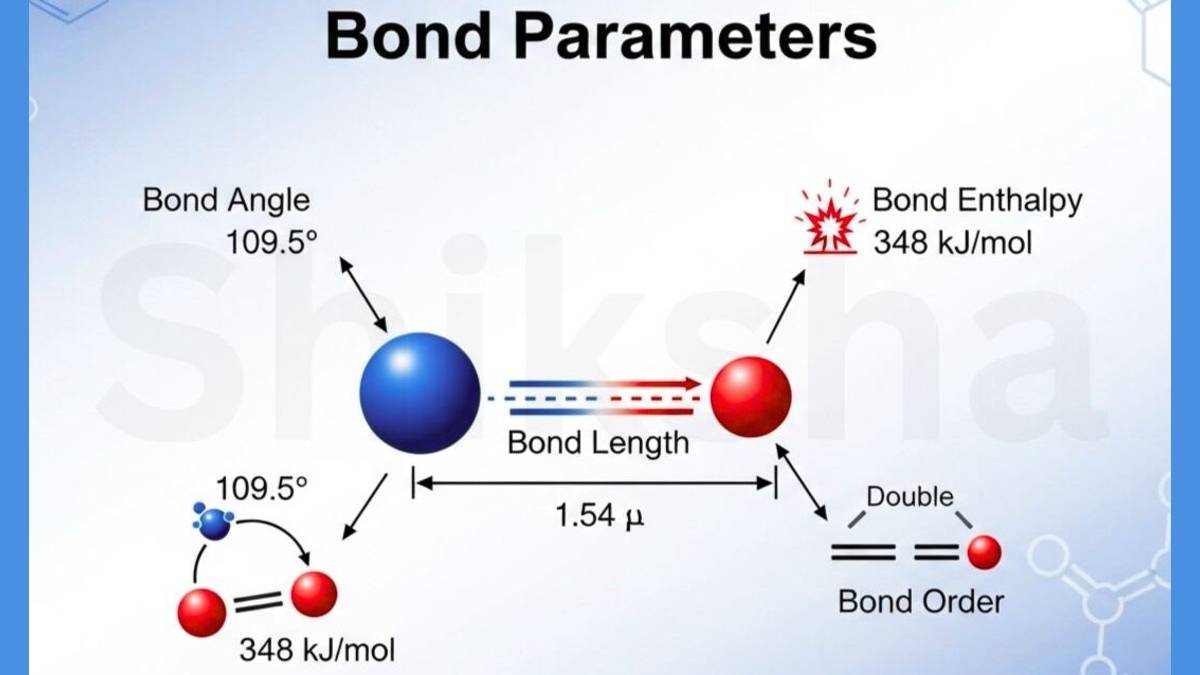
The Chemical bonds hold atoms together to form molecules of chemical compounds. There are several characteristics of chemical bonds that define their strength, length, and behavior. These characteristics are known as bond parameters.
These bond parameters include bond length, bond angle, bond enthalpy, bond order, bond polarity, and dipole moment. The NCERT notes of all other topics of this chapter, such as Lewis theory, Valence Bond theory, and more, are also provided. These bond parameters influence the physical and chemical properties of chemical compounds.
The Bond parameters are affected by factors such as atomic size, electronegativity, hybridization, and lone pairs. You must have a good understanding of bond parameters to get better scores in CBSE and competitive exams. Read the complete article below.
- Key Bond Parameters
- Bond Length
- Bond Angle
- Bond Enthalpy
- Bond Order
- Bond Polarity
- Dipole Moment
- Summary of Bond Parameters
- Study Materials for CBSE Class 11
- Class 11 Chemistry Chapter-wise Notes for CBSE
- Questions related to Bond Parameters
Key Bond Parameters
There are the following characteristics of chemical bonds are considered bond parameters. You can check the bond parameters discussed below.
- Bond length
- Bond Angle
- Bond order
- Bond Enthalpy
- Bond Polarity
Bond Length
When two atoms bind together to form a molecule, there is a certain distance between the nuclei of the two atoms. In general, this distance between nuclei in an equilibrium condition is considered as Bond Length. In covalent bonds, the bond length is nearly equal to the sum of the atomic radii of the two participating atoms. As explained in the below given NCERT figure, for two atoms A and B, the atomic radii are and respectively. The bond length will be equal to the sum of and .
Bond length = +
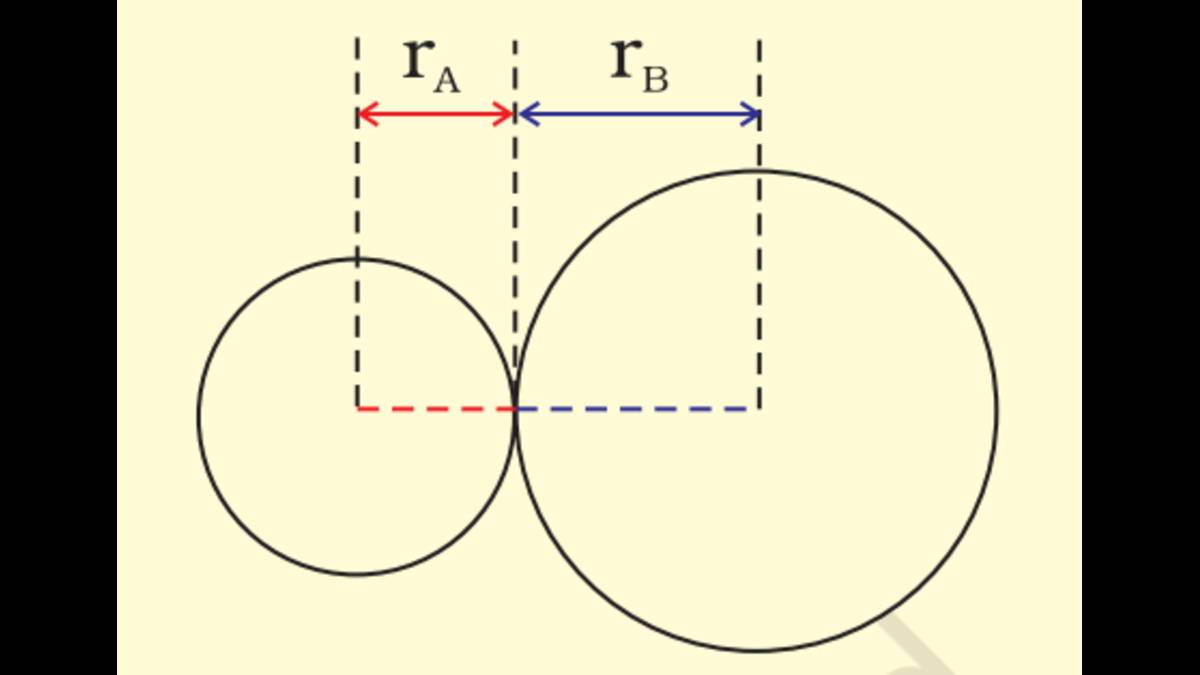
Bond Length (NCERT image)
Important Points related to Bond Length
- It depends on three main key factors: the size of atoms, the bond order, and the bond type.
- The bond length decreases from single to triple bonds. Bond length is maximum for a single bond and then decreases as the number of bonds (Bond order) increases.
- The shorter the bond length, the stronger it will be.
- It is measured in picometres (pm) or angstroms (Å). {1 Å = 100 pm}
- It is measured using X-ray diffraction or spectroscopic methods.
Bond Angle
When atoms combine to form a molecule, they form a certain geometrical shape. The angle between two adjacent bonds, which is important to hold that shape, is known as bond angle.
As per the NCERT definition," the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion."
Key Points related to Bond Angle
-
It helps in predicting the shape of the molecule.
-
The bond angle of any molecule depends on its hybridization of the central atom, the electronegativity of surrounding atoms, and the number of lone pairs of electrons.
- Hybridization determines the bond angle due to the specific spatial arrangement of orbitals.
- In sp hybridization, the bond angle is 180°.
- In sp² hybridization, the bond angle is 120°.
- In sp³ hybridization, the ideal bond angle is 109.5°.
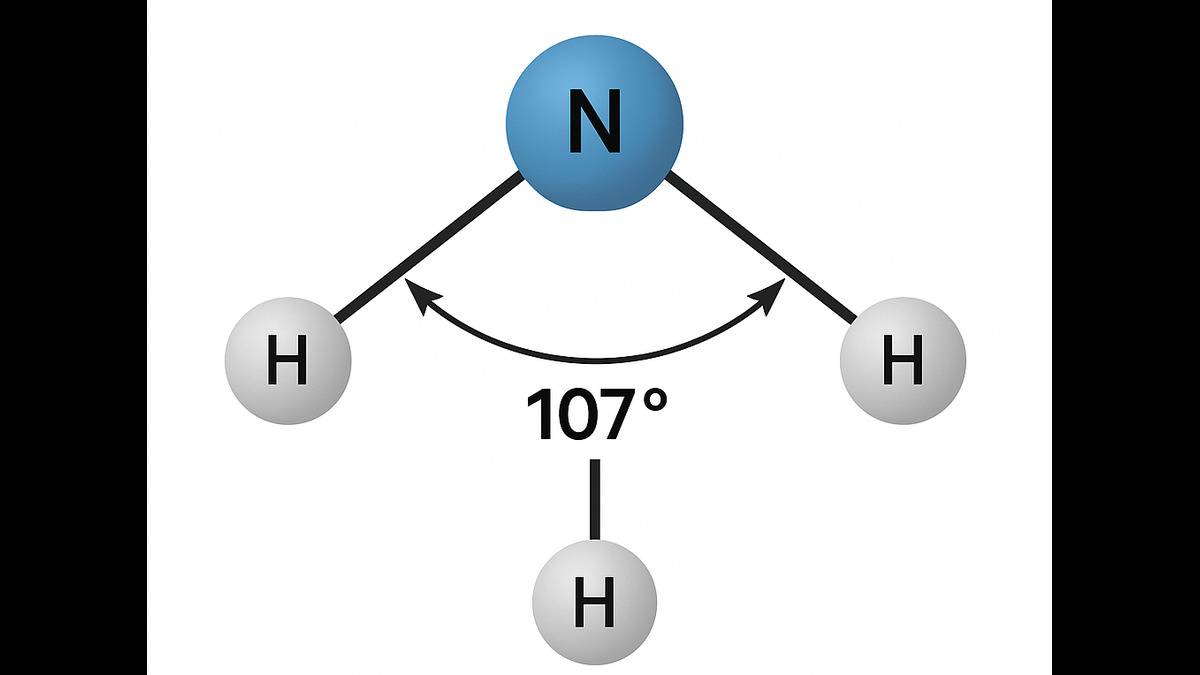
- Lone pairs generally have more electrostatic repulsion, which reduces bond angles, such as in the case of NH₃; even after sp³ hybridization, the bond angle is 107°.
- The electronegative atoms pull bonding electrons closer, so the higher the electronegativity, the smaller the bond angles will be.
Bond Enthalpy
All chemical bond needs some amount of energy to break free. Bond enthalpy defines the amount of energy required to do so. The bond enthalpy of any chemical bond will be equal to breaking one mole of the bond in the gaseous state.
As per the NCERT definition," The amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state is called bond enthalpy."
Key Points related to Bond Enthalpy
- The stronger the bond is, it will require more energy to break in a gaseous state, which means a higher bond enthalpy.
- It is measured in kJ mol⁻¹.
-
A formula to estimate enthalpy changes in reactions:
- In polyatomic molecules, the term mean bond enthalpy is used instead of bond enthalpy. It is obtained by dividing the total bond dissociation enthalpy by the number of bonds broken.
Bond Order
As per the Lewis theory of formation of covalent bonds, the number of bonds formed between the two atoms in a molecule is called the Bond Order. For Example, the bond order of diatomic nitrogen N≡N is three where whereas the bond order of diatomic Oxygen O=O is two.
Key Points related to Bond Order
- As we increase in bond order, the bond enthalpy increases, and the bond length decreases.
- Formula for bond order in Molecular Orbital Theory:
-
-
: Number of electrons in bonding orbitals
-
: Number of electrons in anti-bonding orbitals
-
Bond Polarity
In general reality, no compound has either a completely covalent or an ionic bond. When the shared electron pair between the two atoms gets displaced more towards the higher electronegative atom, the resultant covalent bond is a polar covalent bond. Basically, the difference in electronegativity of the two atoms sharing the pair of electrons gives rise Polarity of bonds.
Key Points related to Bond Polarity
-
A higher electronegative atom pulls shared electrons, so it develops a partial negative charge.
-
The Less electronegative atom develops a partial positive charge.
- Examples:
-
-
H–Cl: polar (Cl more electronegative)
-
H–H or Cl–Cl: non-polar (same atoms)
-
Dipole Moment
Dipole moment of a bond is calculated as the product of charge (q) and distance (r) between the charges: it is denoted as
Key Points of Dipole Moment
-
Units: Debye (D)
-
It is a Vector quantity.
-
Symmetrical molecules are non-polar because their dipole moments cancel out.
- Asymmetrical molecules are polar because there is a dipole moment.
- Examples:
-
-
HCl → 1.08 D (polar)
-
CO₂ → 0 D (linear, non-polar despite polar bonds)
-
NH₃ → 1.47 D
-
Summary of Bond Parameters
Study Materials for CBSE Class 11
Class 11 Chemistry Chapter-wise Notes for CBSE
Questions related to Bond Parameters
Commonly asked questions
Why the bond length in NO? is shorter than in NO?
The NO (Nitric Oxide) has 11 valence electrons while the NO? (Nitrosonium ion) has 10 valence electrons due to removal of one antibonding electron. Due to this, the bond order of NO increases from? 2.5 to 3 in the NO? (Nitrosonium ion).
As per the NCERT, since the bond order of NO? (3) is higher than that of NO (2.5), the bond in Nitrosonium ion is stronger bond and the stronger the bond, the shorter bond length.
Hence bond length in NO? is shorter the NO.
Explain why the bond angle in is larger than in ?
The reason why bond angle is larger in than are given below.
- In , there is one lone pair on the nitrogen atom increases repulsion, while the lone pair on phosphorus is in a higher energy orbital and causes less repulsion.
- Nitrogen is a small and electronegative atom whereas phosphorus is larger and less electronegative than nitrogen.
- In , the bonding orbitals are nearly pure p-orbitals, which are less directionals–p hybridization, on the other hand, the bond pairs remain fairly directed, leading to a larger bond angle.
Hence, the bond angle in is about 107° ( less than the ideal tetrahedral 109.5°) due to lone pair presence and highly electronegative atom, and the bond angle in PH? ( 93.5°) is much smaller due to less repulsive lone pair, and less electronegative atom.
Hence, the bond angle in (107°) is larger than in (93.5°).
Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics