There have been many attempts to explain the formation of chemical compounds and chemical bonding in these compounds. These attempts include important theories, including valence bond theory, molecular orbital theory, and VSEPR theory. The VBT deals with atomic orbitals and their overlapping with nearby different types of atomic orbitals and resulting in covalent bonds.
The process of two atomic orbitals overlapping to create a new type of hybridized orbital is known as hybridization. Generally, atomic orbitals of the same energy level participate in Hybridization. Linus Pauling proposed the concept of hybridization and which determines the shape and bonding patterns. The concept of hybridization is an extension of valence bond theory that helps us understand bond formation, bond energies, and bond lengths.
Shiksha offers complete study material for the Class 11 chemistry chemical bondign and molecular structure chapter with completely free PDF. Our NCERT Notes for all topics of class 11 chemistry will help you develop the fundamental understanding of chemistry and other science stream subjects. Read the article below;
- What is Hybridization?
- Key Features of Hybridisation
- Steps to Determine Type of Hybridization
- Types of Hybridization
- Hybridisation and VSEPR Theory
- Common Mistakes and Tips to Avoid Mistakes
- How to Solve JEE Main Questions of Hybridization
- Study Materials for Class 11 (NCERT)
- Class 11 Chemistry Chapter-wise CBSE Notes
- Frequently Asked Problems
What is Hybridization?
During the formation of covalent bonds, atomic orbitals overlap according to the valence bond theory. The process of combining atomic orbitals due to overlapping to form newly hybridised orbitals is called hybridization. The same energy level atomic orbitals usually participate in hybridization. These hybrid orbitals have distinct shapes and orientations based on overlapping orientation. For examples, in methane , the carbon atom undergoes hybridisation to form four equivalent bonds with hydrogen, despite having different types of atomic orbitals ( 2 s and 2 p ).
There are three key things involved in hybridization process:
- Overlapping of atomic orbitals of similar energy level to form hybrid orbitals.
- The energy of hybrid orbitals gets redestributed as an average of the original orbitals, making them degenerate.
- Geometric Arrangement of hybrid orbitals determines the molecule's shape, such as linear, tetrahedral, or trigonal planar.
Key Features of Hybridisation
Here are the key features of the hybridization, which you must remember during the exams. Read below:
- Atomic orbitals with equal energies take part in the hybridization process.
- Hybrid orbitals have equivalent energy, which ensures stable bond formation.
- Hybrid orbitals overlap with orbitals of other atoms head-to-head to form sigma bonds, which are strong and directional.
- Hybrid orbitals overlap with orbitals of other atoms sideways to form pi bonds, which are weaker.
- In many compounds like or hybrid orbitals may contain lone pairs, which affect the molecular geometry.
Steps to Determine Type of Hybridization
You can follow these simple steps to determine the type of hybridisation in a chemical compounds along with its molecular structure. Read below.
- Step 1: Find out the electronic configuration of all the elements taking part in the formation of compound. (For example, in case of NH₃, do the elctronic configuration of Nitrogen and Hydrogen.)
- Step 2: Calculate the number of electrons in the valence shell and the valence shell orbital (s,p,d or f).
- Step 3: Calculate the steric number and orbitals overlapping to form the compound.
- Step 4: Match the steric number with the associated molecular geometry, use the table given below for ease.
Table for Identification of Hybridization and Molecular Geometry
| Steric Number | Hybrid Type | Orbitals Mixed | Geometry |
|---|---|---|---|
| 2 | sp | 1 s + 1 p → 2 sp | Linear |
| 3 | sp² | 1 s + 2 p → 3 sp² | Trigonal planar |
| 4 | sp³ | 1 s + 3 p → 4 sp³ | Tetrahedral |
| 4 | sp²d (square planar) | 1 d + 1 s + 2 p → 4 dsp² | Square planar |
| 5 | sp³d (dsp³) | 1 s + 3 p + 1 d → 5 sp³d | Trigonal bipyramidal |
| 6 | sp³d² | 1 s + 3 p + 2 d → 6 sp³d² | Octahedral |
| 7 | sp³d³ | 1 s + 3 p + 3 d → 7 sp³d³ | Pentagonal bipyramidal |
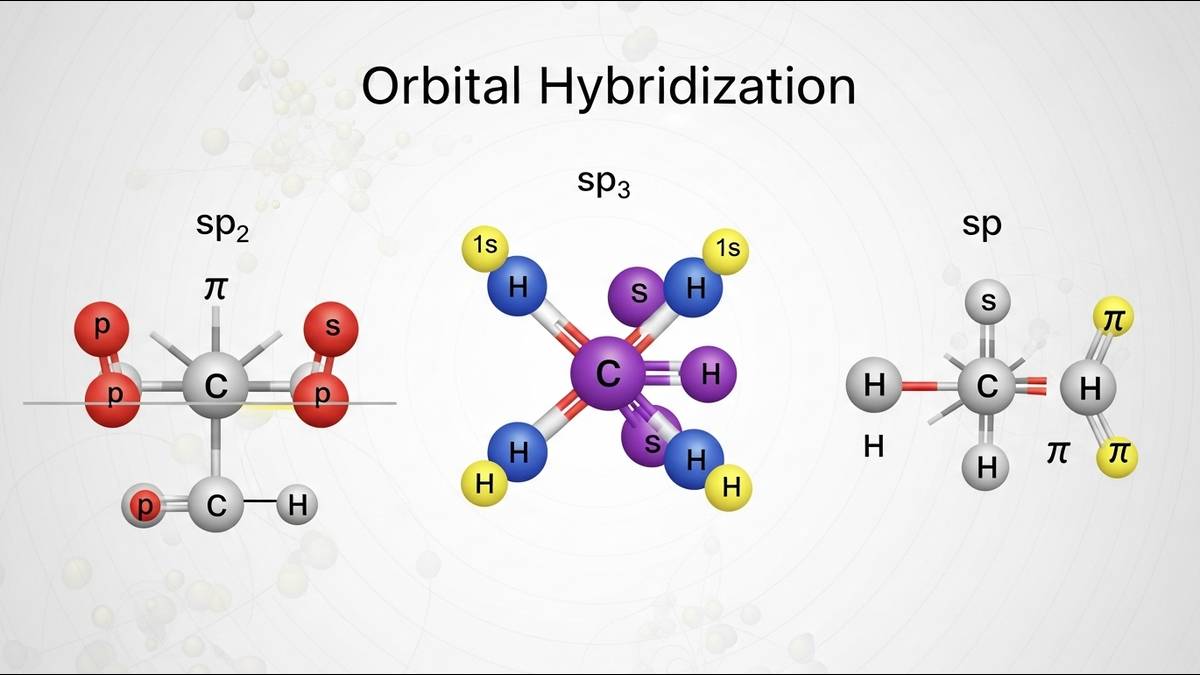
Types of Hybridization
There are various type of hybridised orbitals and hybridization based on orbital orientation. Read complete information realted to the types of the hybridization along with self-explanatory diagrams below.
-
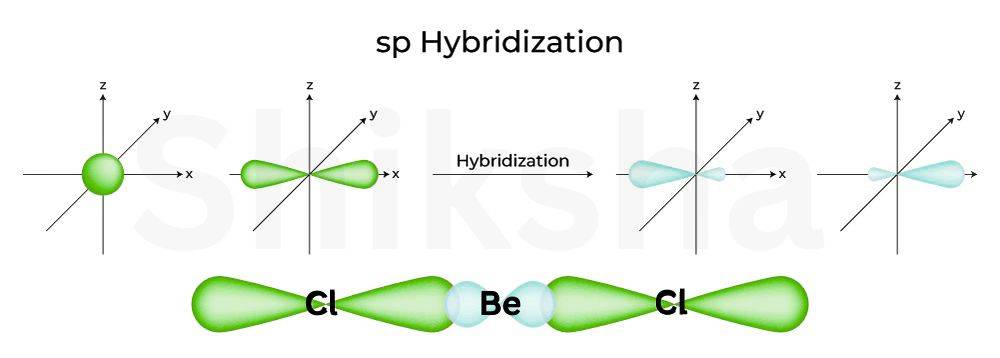
sp Hybridisation
The sp hybridisation involves overlapping of one s and one p orbital to form two sp hybrid orbitals. This hybrid orbital will be linearlly oriented apart. The hybrid orbital contains one electron available for bonding.
Comibation: 1s + 1p orbital at 180° angle, linear overlapping
Example: We can take the example of (beryllium chloride). Beryllium has two valence electrons . To form two bonds, one 2 s electron is promoted to a 2 p orbital, and the 2 s and 2 p orbitals hybridise to form two sp orbitals. These overlap with chlorine's p orbitals, forming a linear molecule (Cl-Be-Cl).
-
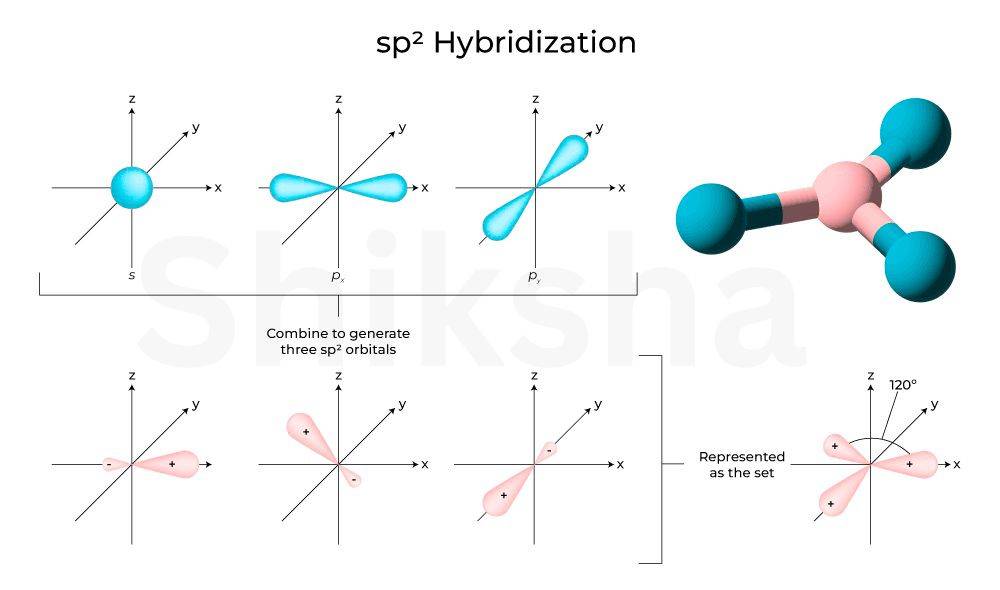
Hybridisation
In hybridization, one s and two p orbitals overlap to combine and form three hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar fashion ( bond angles). This is common in molecules with three electron domains around the central atom.
Comibation: overlapping of 1s and 2p orbital at 120° angle
Example: Taking the example of (boron trifluoride). Boron's 2 s and 2 p orbitals hybridise to form three orbitals, each forming a sigma bond with fluorine's orbital. The molecule adopts a trigonal planar shape.
-
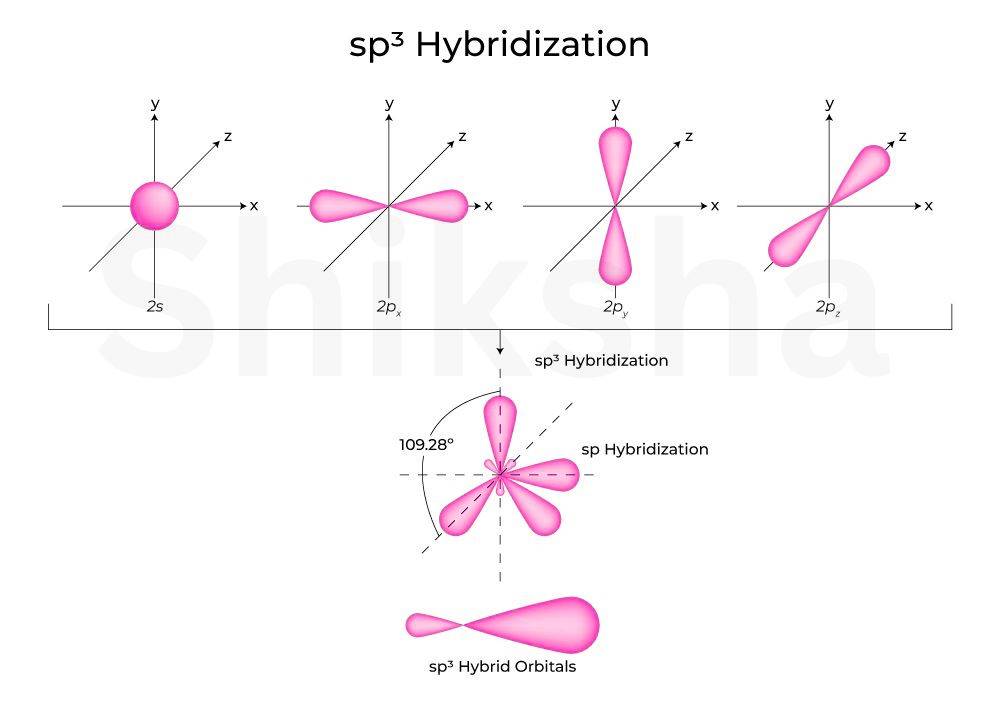
Hybridisation
In hybridisation one s and three p orbitals overlap and combine to form four hybrid orbitals. The orientation of hybrid orbitals is tetrahedral (109.5 ° bond angles). This is commonly found in 4 single bond compound attached to a central atom like methane.
Comibation: overlapping of 1s and 3p orbital at 109.5° angle
Example: The most prominent example is (methane). Carbon's 2 s and 2 p orbitals hybridise to form four orbitals, each overlapping with hydrogen's 1s orbital, resulting in a tetrahedral structure.
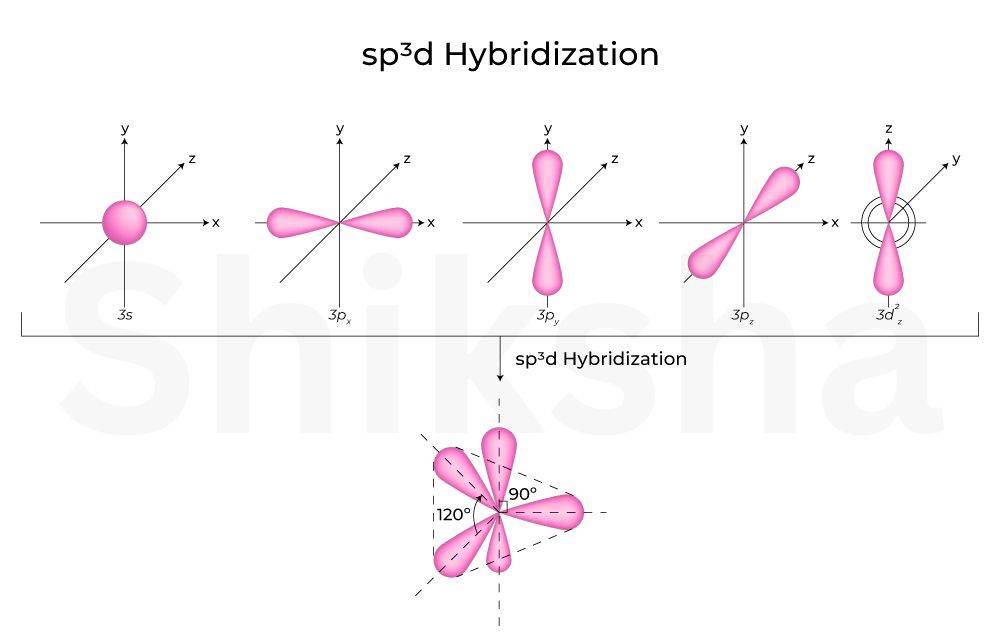
-
Hybridisation
The is complex type hybridization structure. This involves not just s and p but also te d orbital. In hybridisation, one s, three p, and one d atomic orbitals overlap and combine to form five hybrid orbitals. You can check the orbital overlapping and diagram below.
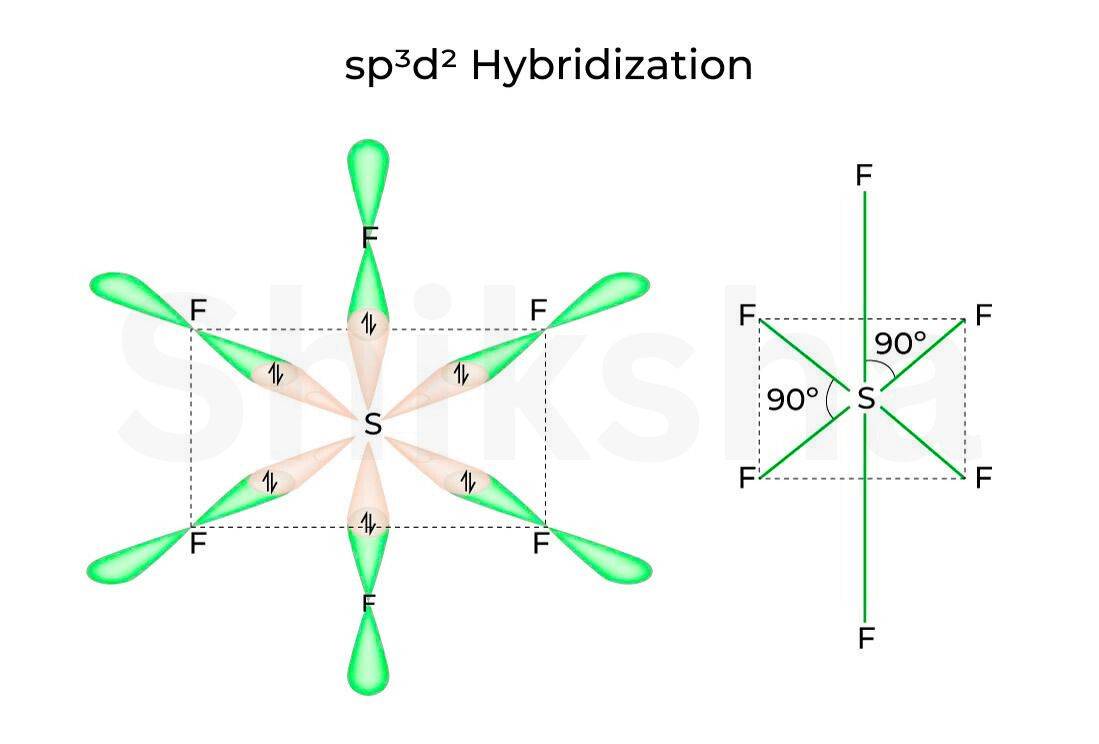
-
Hybridization
The has also a complex type hybridization stats, as both of atoms involve d orbital..six hybrid orbitals form instead of 5 like hybridization. This type of hybrid orbital has an octahedral geometry. The shape of these orbitals will be a trigonal bipyramidal shape. check the diagram below.
Examples: Phosphorus pentachloride (PCl₅) uses one 3s, three 3p, and one 3d orbital to form five orbitals.
Sulphur hexafluoride(SF₆) hybrid orbitals form six orbitals, with an octahedral geometry.
Hybridisation and VSEPR Theory
Here are the clear differences in hybridisation and VSEPR theory.
| Features | Hybridisation | VSEPR Theory |
|---|---|---|
| Fundamental Concept | Combination of Atomic Orbitals | Electron Pair Repulsion |
| Key Point | Orbital Overlapping | Electron Pair Repulsion |
| Explanation | Bond Formation | Molecular Geometry |
| Participating Element | s, p, d orbitals | Electron Domains |
| Example | CH₄ (sp³), BF₃ (sp²), BeCl₂ (sp) | CH₄ (tetrahedral), NH₃ (trigonal pyramidal), H₂O (bent) |
Common Mistakes and Tips to Avoid Mistakes
Many students fall for the mistake due to urgency or the need to quickly solve problems. Here are a few important areas where you can make mistakes. You can check the point of mistake and tips to avoid them as well. Read below.
- Calculating Steric Number: This is important to understand that the steric number is the sum of all electron domains or groups, rather sum of all electron pairs and the individual number of bonds. You need to carefully examine the electron domains, especially in molecules with multiple lone pairs or resonance involvement or multiple bonds.
- Confusing σ vs π Bonds Counting: When students count individual bonds instead of electron domains, they miscalculate the steric number. For example, if you are treating a double bond as two electron pairs instead of one electron domain, It will lead to wrong classification of hybridization.
- Resonance and Delocalization Effect: Some atoms, such as nitrogen and oxygen, show resonance in certain conditions, which changes the hybridization state. You must carefully understand the concept of resonance and delocalization of electrons as per molecular orbital theory to avoid these mistakes.
Tips to Avoid These Mistakes
- You should draw Lewis structures to identify the bond pairs, lone pairs, σ domains, and other electron domains.
- Count the Steric Number very carefully, especially count double/triple bonds as a single entity.
- Always check for resonance in the atom while overlapping; Forexample if there is delocalization of an electron, the central atom often uses sp² hybridization to leave a p orbital free.
How to Solve JEE Main Questions of Hybridization
JEE Main questions frequently include Hybridisation concepts, molecular geometry, bond angles, and chemical properties. You can check the type of questions asked based on this topic below:
- Question related to the prediction of geometry
- Question based on bond angles of some specific compounds like water, methane.
- Bond strength and effects of hybridization in bond strength-based questions
Study Materials for Class 11 (NCERT)
Class 11 Chemistry Chapter-wise CBSE Notes
Frequently Asked Problems
Commonly asked questions
Determine the hybridisation and geometry of the central atom in .
Xenon has 8 valence electrons. In , it forms two bonds with fluorine, leaving three lone pairs. The steric number =2 bonds +3 lone pairs .
As per the VSEPR Theory the electron? pair geometry due to SN = 5 will be trigonal bipyramidal. The hybridization type will be hybridisation. However, the three lone pairs occupy equatorial positions, resulting in a linear molecular geometry.
Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics