Many compounds are formed by overlapping and combining atoms of the same elements. You know O₂, N₂, Cl₂, and many other such chemical compounds. Those Chemical Compounds that have two atoms of the same element are called Homonuclear Diatomic Molecules.
The Chemical Bonding of these Homonuclear diatomic molecules is explained using molecular orbital theory (MOT). The Molecular orbitals of these homonuclear diatomic molecules are determined using the electron configuration of the atomic orbitals. The Chemical bonding, as per Molecular orbitals overlapping, determines various bond parameters such as bond order, bond length, bond energy, and magnetic properties of the compound.
You can check multiple concepts involved in the Chemical Bonding and Molecular Structure topic on the Shiksha pages. We have compiled detailed notes with conceptual depth, practice problems, explanatory diagrams, and more. Read the article below for complete information.
- What are Homonuclear Diatomic Molecules (NCERT Definition)?
- Formation of Homonuclear Diatomic Molecules
- Important Homonuclear Diatomic Molecules
- Magnetic Properties of Homonuclear Diatomic Molecules
- Key Homonuclear Diatomic Molecules and Important Properties for JEE Main
- Factors Influencing Bonding
- Summery: Important Homonuclear Diatomic Molecules
- Tips for JEE Main Exam
- Study Materials for CBSE Class 11
- Complete Class 11 Chemistry Chapter-wise Notes
- Important Questions Related to Homonuclear Diatomic Molecules
What are Homonuclear Diatomic Molecules (NCERT Definition)?
As per NCERT definition, "Homonuclear diatomic molecules are molecules that consist of two atoms of the same element bonded together."
Chemical bonding in homonuclear diatomic molecules is explained through the linear combination of atomic orbitals (LCAO) to form molecular orbitals. The LCAO is discussed in detail in our Molecular Orbital Theory Notes. These molecules are often formed by non-metal elements. These molecules are bonded by covalent bonds. For Example: H₂, N₂, O₂, F₂, Cl₂, Br₂, I₂, etc.. You can check the figure below. We have attached the figure of O₂, which is a homonuclear diatomic molecule.
Formation of Homonuclear Diatomic Molecules
Both Valence Bond Theory and Molecular Orbital Theory explain the formation of these molecules. VBT offers a simpler explanation, but it has a few drawbacks in predicting magnetic properties and others. MOT, on the other hand, is more accurate and predicts the magnetic nature of molecules. According to the MOT theory, two atoms follow several steps to form molecular orbitals. You can review the steps for easier understanding.
- Atomic orbitals of the same element overlap and combine to form a molecular orbital.
- When two atomic orbitals overlap, they fill electrons in molecular orbitals in the increasing order of orbital energy.
- These Atomic orbitals and molecular orbitals both follow the Aufbau principle and Hund’s Rule to pair the electrons.
- Those HDMs that have unpaired electrons left in the molecular orbital are paramagnetic.
- The HDMs that have only paired electrons are diamagnetic.
- The Bond order is calculated using the following formula:
-
- Bond Order: Calculated as (number of bonding electrons-number of antibonding electrons).
Forces between Homonuclear Diatomic Molecules
Different types of forces exist between homonuclear diatomic molecules. These forces depend on various variables, including temperature, pressure, molecular distance, and the nature of bonding. Here are the important forces between these molecules.
- Covalent bonding (electrostatic Attraction Force)
- Vanderwaals Force
- Dipole-Dipole Interaction
- London Force
Important Homonuclear Diatomic Molecules
-
Oxygen Molecule (O₂)
The oxygen molecule is made up of two oxygen atoms. Two atomic orbitals combine to form lower lower-energy molecular orbital of the oxygen molecule. As a result, these two oxygen atoms form a covalent bond.
-
- The electronic configuration of the Oxygen molecule is given as, O2: (σ1s)2 (σ*1s)2 (σ2s)2 (σ *2s)2 (σ2pz) 2 (π2p2x= π 2p2y) (π*2p1x= π*2p1y)
- The bond order of the Oxygen molecule = , which means the oxygen atom will have a double covalent bond.
- O₂ also has unpaired electrons as per the electronic configuration, which makes it a Paramagnetic molecule.
-
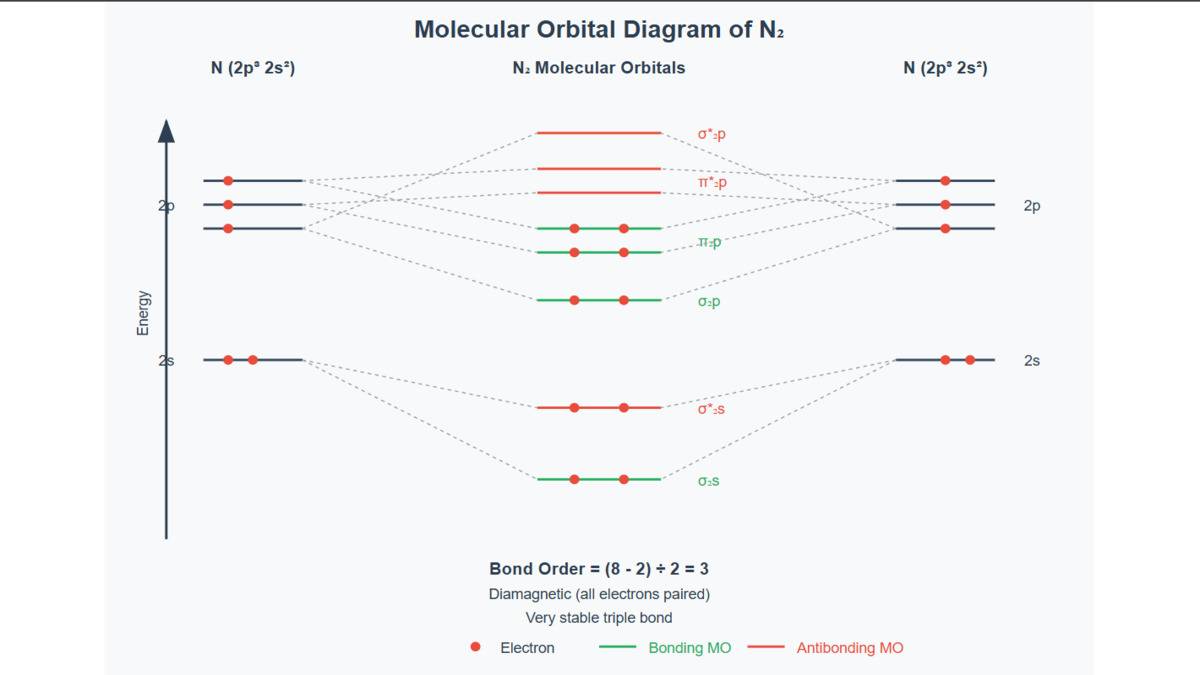
Nitrogen Molecule
Two nitrogen atoms combine their atomic orbitals to form a diatomic nitrogen molecule. When the atomic orbitals of nitrogen combine, they form bonding and antibonding molecular orbitals. During overlapping, they share three pairs of electrons to form a triple covalent bond.
-
- The electronic configuration of the nitrogen molecule is: N₂: (σ1s)2 (σ*1s)2 (σ2s)2 (σ *2s)2 (σ2px) 2 =(π2p2y) (π 2p2z)
- The bond order of the nitrogen molecule = ½ × (10 − 4) = 3, which means the N₂ molecule has a triple covalent bond.
- All the electrons in the diamagnetic molecules are paired, so N₂ is a diatomic compound.
- Hydrogen Molecule (H₂)
When two hydrogen atoms come close, they share their single electron to get the state of inertness. The formation of the molecular orbital of H₂ is due to the overlapping of atomic orbitals and the sharing of electrons. H₂ is a member of the diatomic molecule family.
-
- The electronic configuration of the Hydrogen (H₂) molecule is : (σ1s)²
- The bond order of the nitrogen molecule = , which means the H₂ molecule has a single covalent bond.
- H₂ has no unpaired electrons left in the molecular orbital, making it a diamagnetic molecule.
-
Lithium Molecule (Li₂)
Like other homonuclear diatomic molecules, Lithium also combines two os its atoms to form a molecule (Li₂). The individual atomic orbitals of Lithium overlap to form a molecular orbital of Li₂. Lithium has only 3 electrons in which 1 electron in the 2s shell roams free, which is shared ot form a covalent bond.
-
- The Electronic confihuration of Lithium Molecule (Li₂): (σ1s)² (σ*1s)² (σ2s)²
- The bond order of the Lithium molecule = ½ × (4 − 2) = 1, which means the Li₂ molecule has a single covalent bond.
- All electrons of the Lithium molecule are paired; that's why Li₂ is a diamagnetic molecule.
-
Chlorine Molecule (Cl₂)
The chlorine molecule consists of two chlorine atoms, which combine to form a molecular orbital. Each chlorine atom has 17 electrons, and when the atomic orbitals of chlorine overlap, one pair of valence electrons is shared as per the MOT theory. Each Cl shares one electron to form Cl–Cl single covalent bond.
- The Electronic confihuration of Chlorine Molecule Cl₂ : (σ1s)² (σ1s)² (σ2s)² (σ2s)² (σ2pz)² (π2px)² = (π2py)² (π2px)² = (π2py)² (σ2pz)² (σ3s)² (σ3s)² (σ3pz)² (π3px)² = (π3py)² (π3px)² = (π3py)² (σ*3pz)²
The bond order of the chlorine molecule = ½ × (2 − 0) = 1. There is a single covalent bond between Cl₂. - Since all electrons are paired, Cl₂ is diamagnetic molecule.
Magnetic Properties of Homonuclear Diatomic Molecules
As per the explanation in the MOT theory, the magnetic nature of the molecule depends on the presence of the unpaired electron in the molecular orbital of the compound.
- Diamagnetic: If all electrons in the molecular orbital are paired.
- Paramagnetic: If there are Unpaired electrons present in the molecular orbital.
Key Homonuclear Diatomic Molecules and Important Properties for JEE Main
The following molecules, commonly tested in JEE Main, are analyzed using MO theory:
(Hydrogen Molecule)
- Electron Configuration: Each H atom contributes one 1s electron.
- Bond Order: (single bond).
- Properties: Bond length=74pm, bond energy=432 kJ/mol, diamagnetic (no unpaired electrons).
(Nitrogen Molecule)
- Electron Configuration: Each N atom has 7 electrons (total 14).
- Bond Order: (triple bond).
- Properties: Bond length=110pm, bond energy=945 kJ/mol, diamagnetic.
(Oxygen Molecule)
- Electron Configuration: Each O atom has 8 electrons (total 16).
- Bond Order: (double bond).
- Properties: Bond length = 121 pm, bond energy=495 kJ/mol, paramagnetic.
(Fluorine Molecule)
- Electron Configuration: Each F atom has 9 electrons (total 18).
- Bond Order: (single bond).
- Properties: Bond length=144pm, bond energy=495 kJ/mol, diamagnetic.
Factors Influencing Bonding
- Bond Order: Higher bond order (e.g., ) results in shorter bond length and higher bond energy.
- s-p Mixing: Affects MO energy levels in and , altering the order of and orbitals compared to .
- Electron Repulsion: In , lone pair repulsion reduces bond strength.
Summery: Important Homonuclear Diatomic Molecules
| Molecule |
Bond Order |
Bond Length (pm) |
Bond Energy (kJ/mol) |
Magnetic Propert |
|---|---|---|---|---|
| 1 |
74 |
436 |
Diamagnetic |
|
| 3 |
110 |
945 |
Diamagnetic |
|
| 2 |
121 |
498 |
Paramagnetic |
|
| 1 |
142 |
159 |
Diamagnetic |
Tips for JEE Main Exam
Study Materials for CBSE Class 11
Complete Class 11 Chemistry Chapter-wise Notes
Important Questions Related to Homonuclear Diatomic Molecules
Commonly asked questions
As per the molecular orbital theory, two atomic orbitals of oxygen atoms combine to form lower-energy molecular orbital of the oxygen molecule.
- Bond order of the Oxygen molecule = , which means a double covalent bond.
- Since O? also has unpaired electrons, so it is a Paramagnetic molecule.
You can read complete information in our homonuclear diatomic molecules notes bases on class 11 chemistry.
The stability of the bond depends on various bond parameters including the bond length and bond energy. In general the shorter the bond, the lesser the bond energy will be, this leads to the stable molecule.
Since the bond order and energy of hydrogen molecule is lesser than the oxygen molecule, it is be more stable.
Molecule | Bond Order | Bond Length (pm) | Bond Energy (kJ/mol) |
|---|---|---|---|
1 | 74 | 436 | |
2 | 121 | 498 |
Chemistry Chemical Bonding and Molecular Structure Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics