German physicist, Werner Heisenberg, and French physicist, de Broglie, between 1924 and 1927, were two main figures who paved the way towards the quantum mechanical model of the atom.
The classical models of the atom, up until Bohr’s model, were huge discoveries of the early 20th century. The earlier atomic models, however, couldn't explain that electrons didn’t have orbits like planets around the sun. Rutherford would later explain how electrons orbit around the nucleus, but one major shortcoming was to rely on classical physics, which made him consider that the electrons would spiral into the nucleus.
Bohr defined quantised energy levels of electrons, refining the views of Planck, Maxwell, Einstein, Balmer, and Rydberg. The main limitation of the atomic model of Bohr was it could not explain the behaviour of atoms for more than one electron.
For the next step in the evolution, we have to thank the Heisenberg Uncertainty Principle and de Broglie’s hypothesis.
Aligning with your Class 11 Chemistry syllabus, we have created a more straightforward guide to the developments that led to the quantum mechanical model.
What You’ll Learn
- Why Bohr's model of the atom was incomplete
- The concept of wave-particle duality for matter
- How Heisenberg's Uncertainty Principle changed our view of electron movement and arrangement
- Why Did We Need a New Quantum Model? Limitations of Bohr's Model
- Dual Nature of Matter: de Broglie's Hypothesis
- A Fundamental Limit: Heisenberg's Uncertainty Principle
- Comparing Atomic Models: Bohr vs. Quantum Mechanical Model
- Revision Notes for Chemistry Class 11 NCERT
Why Did We Need a New Quantum Model? Limitations of Bohr's Model
Bohr's model was successful for the hydrogen atom only. It failed to explain complex atomic phenomena.
Bohr's model accurately predicted the spectrum of hydrogen but struggled with multielectron atoms, fine spectral lines, and effects such as Zeeman splitting (in magnetic fields) and Stark splitting (in electric fields).
It also ignored electron wave properties and quantum uncertainties.
Main Limitations of the Bohr Model
- Inapplicable to atoms with multiple electrons due to electron-electron interactions.
- Unable to account for spectral line splitting observed in magnetic or electric fields.
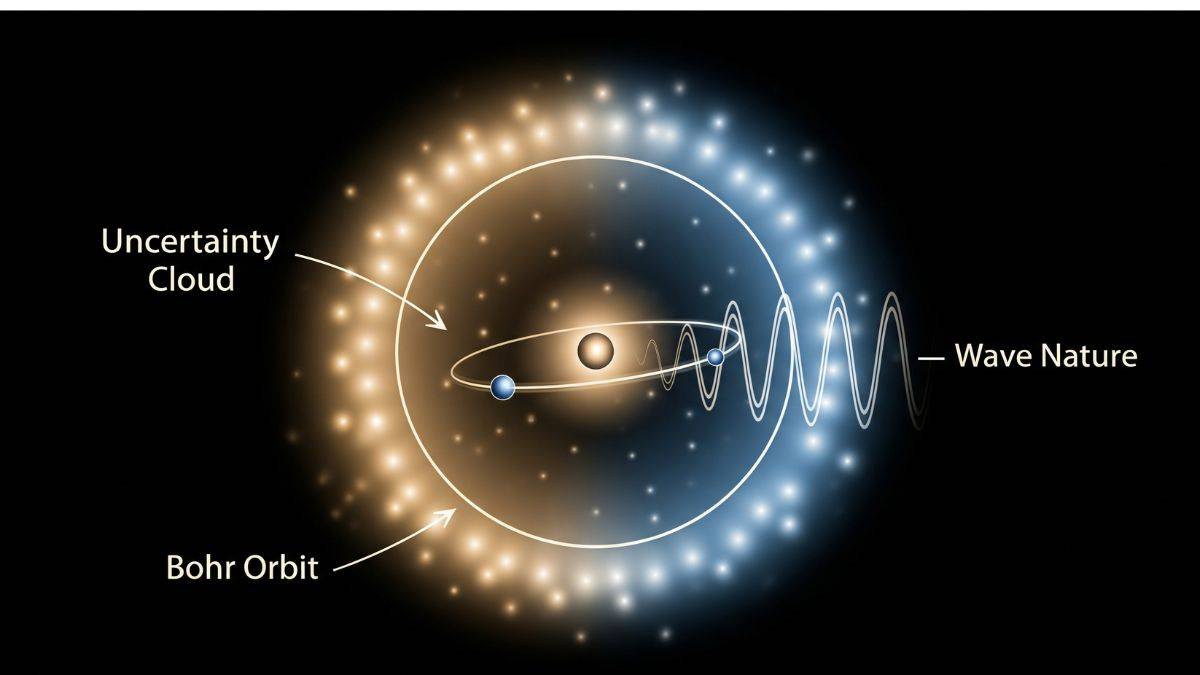
- Assumes fixed orbits, contradicting wave-particle duality.Fails to incorporate Heisenberg's uncertainty principle.
3 Reasons Why Bohr's Atomic Model Didn't Work
Reason 1
Bohr pictured electrons as tiny charged marbles zooming in perfect circles. Nice idea. Problem is, electrons aren’t obedient marbles. They’ve got wave vibes too, which the Bohr model totally ignored.
Reason 2
To say an electron is on a neat orbit, you’d need to know exactly where it is and how fast it’s going at the same time.
Heisenberg rolled in and said: nope, not possible. This is the uncertainty principle in action. Nature basically says “pick one, position or speed, you can’t have both.”
Reason 3
So the Bohr model not only forgot that electrons act like waves. It also broke the biggest rule in quantum physics, the uncertainty principle. That’s like failing the exam and breaking the pencil while doing it.
Dual Nature of Matter: de Broglie's Hypothesis
The dual nature of matter posits that electrons show both particle-like and wave-like properties. Their wavelengths depend on their momentum.
Louis de Broglie (1924) proposed that all matter has wave-like characteristics, with wavelength as expressed by:
where is Planck's constant ( ), is momentum, is mass, and is velocity.
Why is this significant?
- For macroscopic objects (like a baseball), the mass (m) is so large that the wavelength (λ) is tiny and undetectable. On the other hand, for microscopic particles (such as an electron), the mass is extremely small. That makes its wavelength significant and measurable.
- This idea was confirmed in 1927 by the Davisson-Germer experiment. This showed electrons diffracting off a nickel crystal. This behaviour is one of the primary characteristics of waves.
Key Features of de Broglie’s Hypothesis
- For an electron accelerated by a voltage :
where .
- In Bohr's orbits, de Broglie's waves fit as standing waves:
- The wave nature is significant for microscopic particles but negligible for macroscopic objects.
Trivia: It is important to note that using de Broglie's equation, it was Erwin Schrödinger who came up with the equation of quantum mechanics, and also received the prestigious Novel Prize in 1933. Learn more about the Schrödinger Wave Equation in Class 12 Physics.
A Fundamental Limit: Heisenberg's Uncertainty Principle
The uncertainty principle proposes that we cannot simultaneously measure some pairs of physical properties, such as position and momentum, with arbitrary precision.
Werner Heisenberg (1927) formulated:
where is position uncertainty, is momentum uncertainty, and is Planck's constant. Similarly, for energy and time:
This principle invalidates Bohr's precise orbits, favoring probabilistic electron distributions. Key Features:
- Challenges classical mechanics' deterministic view of particle motion.
- Implies electrons exist in probability clouds, not fixed paths.
- Impacts measurements in spectroscopy and quantum calculations.
What do these limitations mean for the atom?
- If an electron were in a fixed orbit, as Bohr suggested, we would know its position and momentum precisely. That isn’t possible with the uncertainty principle.
- The uncertainty principle forces us to abandon the idea of fixed paths. Instead, we can only describe the probability of finding an electron in a specified region in space. This region is often referred to as an "electron cloud" or an orbital.
Comparing Atomic Models: Bohr vs. Quantum Mechanical Model
We should ideally compare atomic levels up to the development of the Bohr Model and then the quantum mechanical model.
| Feature |
Bohr's Model |
Quantum Mechanical Model |
| Electron Path |
Electrons move in fixed, circular orbits. |
Electron position is described by a probability cloud (orbital). |
| Properties |
Position and momentum are definite. |
Position and momentum are uncertain (Heisenberg's Principle). |
| Electron Nature |
Treated as a particle only. |
Treated as having wave-particle duality (de Broglie). |
| Applicability |
Only for single-electron species. |
Applies to all atoms and molecules. |
| Key Concept |
Quantised energy levels. |
Quantised energy levels, orbitals, and quantum numbers. |
Next to read: Quantum mechanical model of the atom.
Further Reading:
NCERT Chapter 2 Chemistry: Structure of Atom
Revision Notes for Chemistry Class 11 NCERT
At Shiksha, you will find more topics that simplify your learning. Browse through NCERT Class 11 Chemistry. Some more topics are below.
Commonly asked questions
Why is it that you cannot observe the wave properties of everyday objects?
De Broglie had the idea that everything that moves has a bit of wave behaviour. Technically, that includes any person, a football, and even a bus. The catch is that for big things, the mass is so large that their wavelength is insanely tiny. It's so tiny it's impossible to notice. That's why you don't see a football spreading out like ripples when you kick it. Electrons though? They're super light, so their wavelengths are big enough for us to actually measure. And when scientists did experiments, such as electron diffraction, the electrons really did behave like waves. That was the proof De Broglie needed to show he was right.
How does the Bohr model contradict the Heisenberg Uncertainty Principle?
Through Heisenberg's Uncertainty Principle, it's proven that you can't pin down where an electron is and how fast it's moving at the same time. The Bohr model did not look into this. The main reason for that thought was that it pictured electrons like little planets that would move in a loop around the nucleus. But that only works if you know both position and speed exactly. Nature doesn't let you do that. Add to it the fact that electrons also behave like waves, and the Bohr model just couldn't keep up. That's why scientists had to move on to the quantum model, which fits way better with how electrons actually act.
Chemistry Structure of Atom Exam
Student Forum
Other Topics under this Chapter
- Isotope Meaning
- Mole Concept
- What are Electrons
- Isotopes and Isobars
- What is a Saturated solution
- Difference between Atom and Ion
- Discovery of Sub Atomic Particles
- Atomic Models
- Developments Leading to Bohr's Model of Atom
- Quantum Mechanical Model of Atom
- Bohr's Model of Hydrogen Atom
- Towards Quantum Mechanical Model of Atom
- Structure of Atom
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test