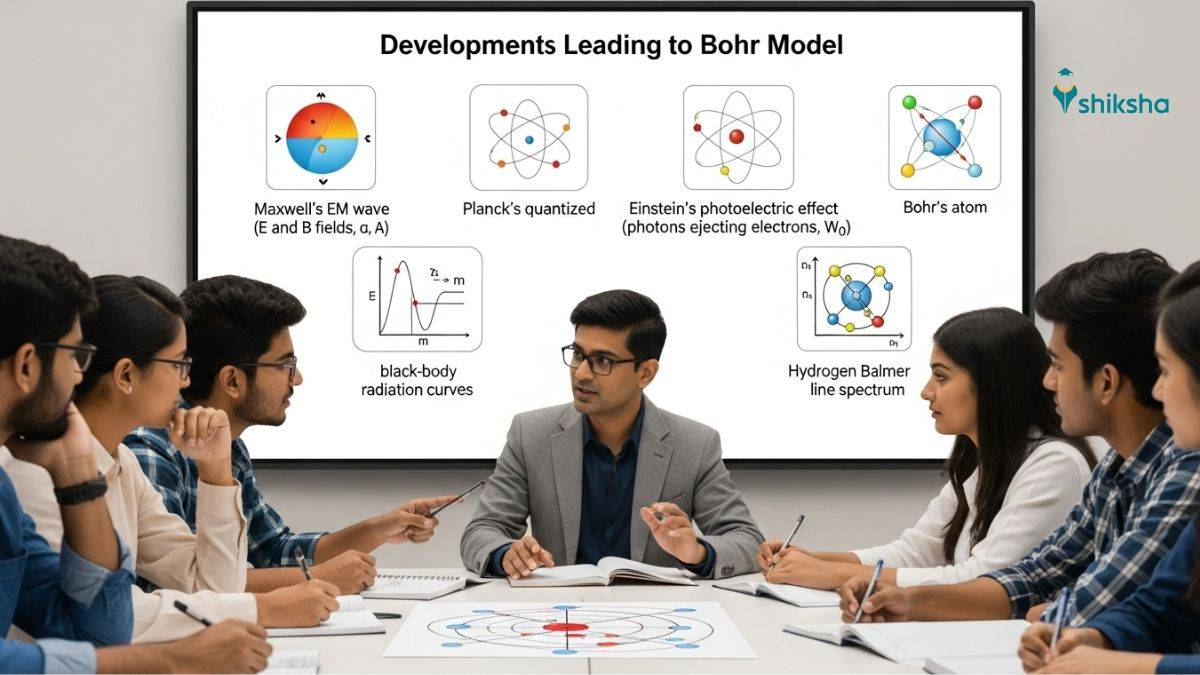
After Rutherford's nuclear model, we have electromagnetic radiation and the quantised nature of energy, which were among the main developments leading to Bohr’s Model. The discovery of subatomic particles, from Thomson's cathode ray experiments to his plum pudding model of the atom, which Rutherford disproved with his alpha-particle scattering experiment, still left scientists wondering about atomic stability and the origin of the spectral lines observed in atoms. But there were some other limitations to the atomic models that you should know before moving on to Bohr's breakthrough. Today, we learn the milestones that laid the groundwork for the atomic structure that we know today. Additionally, we explore how these discoveries bridge the classical and quantum perspectives.
- From Discovery of Subatomic Particles to Atomic Models
- What Were the Main Developments Leading to Bohr Model?
- Dual Nature of Radiation and Matter
- Atomic Spectra and the Road to Bohr
- Outcomes of the Developments for Bohr's Atomic Model
- How to Study Developments Leading to Bohr Model
- JEE-Level Examples
- NCERT Revision Notes Chemistry
From Discovery of Subatomic Particles to Atomic Models
To get the context of the developments of atomic models before Bohr, here is a brief recap.
Subatomic Particles Discovery
The subatomic particles, such as electrons, protons, and neutrons, are fundamental components of atoms, discovered through experiments probing matter's composition.
It’s essential to note that around 1897, J.J. Thomson identified electrons through cathode ray experiments.
These could determine their negative charge and high charge-to-mass ratio -
( ).
Then, we have Goldstein's canal ray studies in 1886. These studies proved that protons are positively charged particles.
Later, in 1932, another scientist, James Chadwick, discovered neutrons, neutral particles, thereby completing the nuclear model.
Features of Subatomic Particles
- Electron: Charge , mass .
- Proton: Charge , mass .
- Charge-to-mass ratio calculations: where is electric field, is magnetic field, and is path radius in Thomson's experiment.
Your upcoming JEE questions may test your knowledge on the properties of subatomic particles or experimental setups. Have a look at the JEE Main Analysis.
Early atomic models proposed structures to explain the arrangement of subatomic particles. This idea evolved from the concept of indivisible atoms to nuclear configurations.
Atomic Models - Thomson to Rutherford
To recap, while reading about early atomic models in the "Structure of Atoms" chapter of the Chemistry CBSE textbook, you must remember that
- Dalton's atomic theory (1808) viewed atoms as indivisible.
- Then we have Thomson's plum pudding model from 1897, which depicted atoms as a positive sphere comprising embedded electrons.
- Later, Rutherford's scattering experiment (1911) introduced the concept of a dense nucleus surrounded by electrons, revealing the atomic structure.
Let’s have a look at the key features of Thomson and Rutherford’s models.
- Thomson's model: Uniform positive charge with electrons, electrically neutral.
- Rutherford's model: Nucleus contains protons and neutrons, electrons orbit at . Rutherford's equation is where is deflection angle, is nuclear charge, is alpha particle energy.
Limitations of the Thomson and Rutherford Models
- Thomson's model failed to explain spectra or scattering.
- Rutherford's model predicted electron collapse due to energy loss.
You can go through the JEE Main question papers. You could find questions on how to contrast these models or focus on scattering experiments.
What Were the Main Developments Leading to Bohr Model?
Niels Bohr improved Rutherford's Nuclear Model by using the studies of radiation and matter interaction during the late 19th century. The major developments in physics that influenced Bohr's theory were:
- Dual character of electromagnetic radiation (wave + particle matter). This was proven by Maxwell's Theory, Planck's Quantum Theory, and Einstein's Photoelectric Effect.
- Experimental evidence from continuous and line atomic spectra. The Balmer's Formula (1885) and the Rydberg Generalisation have been crucial in demonstrating the evidence.
These two developments could answer what Rutherford's model could not explain.
- Stability of the Atom - Rutherford relied on classical physics, which argued that revolving electrons should lose energy as radiation and spiral into the nucleus. Yet an atom was stable.
- Origin of Line Spectra - Elements emit discrete wavelengths or line spectra. Rutherford, however, could not explain why only certain frequencies were observed.
Dual Nature of Radiation and Matter
Maxwell, Planck, and Einstein are the major figures who contributed to explaining the dual nature of radiation and matter, that ultimately helped with Niels Bohr's work.
Wave Nature of Electromagnetic Radiation as Discovered by Maxwell (1870)
James Clerk Maxwell developed the electromagnetic wave theory. This theory states that accelerating charges create electric and magnetic fields that oscillate. These fields travel perpendicular to each other as electromagnetic waves. This theory also explained advanced wave phenomena, such as diffraction and interference. Maxwell could also find out that the electromagnetic spectrum covers a vast range, from long radio waves to short gamma rays, which you will be reading in Class 12 Physics on Electromagnetic waves. Among all these rays, the visible light is just a tiny portion.
Characteristics of Electromagnetic Radiation
- Perpendicular Electric and Magnetic Fields
Oscillating charged particles generate time-varying electric fields that, in turn, induce magnetic fields.
The electric (𝐸) and magnetic (𝐵) components lie at right angles to each other. They are also at right angles to the direction of propagation. Together they form a transverse wave.
- Vacuum Propagation Without Medium
Electromagnetic waves do not need a medium like mechanical waves, such as sound or water waves, do. The components 𝐸 and 𝐵 fields continuously work together, and that allows transmission through the vacuum of space. This is how satellite communication works, as well.
-
Relationship of Wavelength, Frequency, and Light Speed
Maxwell showed this relationship through the equation below that linked wavelength, frequency, and speed of light.
c = νλ
c = speed of light (3.0 × 10⁸ m/s)
ν = frequency (Hz)
λ = wavelength (m)
This equation shows that the speed at which light waves travel is equal to the product of their frequency and their wavelength. When light travels at a constant speed, waves with higher frequencies have shorter wavelengths, and waves with lower frequencies have longer wavelengths. It is foundational for understanding electromagnetic waves, including visible light, radio waves, X-rays, and more.
Particle Nature as Discovered by Planck (1900)
Max Planck, with his hypothesis, answered a couple of confusions among scientists who studied classical wave theory. One of the major problems in classical thought was it could not explain black-body radiation. Such a radiation was a result of emission spectrum of hot objects. The classical wave theory was also not able to describe photoelectric effect.
The Max Planck's Hypothesis says,
Energy exchange between matter and radiation happens in discrete packets (quanta).
E = hν
Where h = 6.626 × 10⁻³⁴ J·s (Planck's constant)
Energy values are quantised like steps on a staircase. So that says that electrons can only gain or lose energy in fixed amounts.
Einstein's Photoelectric Effect (1905)
Einstein had applied Planck's theory to light. He proved that light consists of photons (particles) with energy, E = hν. He said a photon can eject an electron if the 'hν' crosses a threshold energy (work function W₀).
The equation he used was
½mv² = hν - hν₀
Einstein confirmed the dual nature of light in these two ways,
- Shows wave behaviour in propagation
- Shows particle behaviour in interactions with matter
Atomic Spectra and the Road to Bohr
There are four types of spectra to look at.
- Continuous spectrum: This occurs the moment when white light passes through a prism, it produces a continuous band of all colours with no gaps.
- Line spectrum: Unlike continuous spectra, atoms produce only specific wavelengths. That leads to the production of distinct bright or dark lines.
- Emission spectra: When atoms are excited, whether you heat or electrify them, they emit light at discrete wavelengths. These appear as bright coloured lines against a dark background.
- Absorption spectra: When continuous light passes through cool gas, specific wavelengths are absorbed. That produces dark lines in an otherwise continuous spectrum.
Understanding the Hydrogen Spectrum for Evidence
When electric discharge passes through hydrogen gas, it doesn't produce a continuous glow. Instead, it emits light at very specific wavelengths. That creates distinct coloured lines. This was confusing because classical physics couldn't explain why only certain frequencies appeared.
Hydrogen Spectrum Series
The hydrogen spectrum is organised into several series or lines. This was made possible with the studies of Johann Balmer and Johannes Rydberg.
- Lyman series (UV): Transitions ending at n=1
- Balmer series (visible): Transitions ending at n=2 ; These are the lines we can actually see
- Paschen, Brackett, Pfund (IR): Transitions ending at n=3,4,5
Mathematical Pattern Discovery
The wavelengths follow the Rydberg formula
ν̃ = RH(1/n₁² - 1/n₂²)
For the visible Balmer series, we have
1/λ = R(1/2² - 1/n²), n = 3,4,...
Where R = 109678 cm⁻¹ is the Rydberg constant.
Note: This mathematical regularity suggested that electrons exist in discrete energy levels. They do not have continuous orbits as classical physics predicted.
Outcomes of the Developments for Bohr's Atomic Model
The outcomes of the developments fixed two things.
- Quantised orbits prevent electrons from losing energy continuously, fixing the instability problem.
- Discrete jumps between energy levels explain the observed atomic line spectra.
Together, these ideas provided both experimental and theoretical support for Bohr’s postulates.
Next, we have prepared a guide to Bohr's Atomic Model, so that you get into a complete picture of his work and how these developments actually helped.
How to Study Developments Leading to Bohr Model
The development of Bohr's model involved several experiments across electromagnetism and quantum theory conducted by multiple scientists from Maxwell to Einstein. All these could come to the final point of revealing subatomic particles, atomic structure, and the quantised nature of energy. These are basic for JEE Mains, as you will be tested around this topic for various question categories. Have a look at the table below.
| Type of Question |
Example / Focus Area |
| Conceptual |
Why Rutherford’s model failed |
| Derivation/Formula |
Derive the energy of the nth orbit |
| Numerical |
Calculate the wavelength for the transition |
| Application/Assertion-Reason |
Bohr’s postulates and consequences |
| Spectral Series |
Identify the series for the given transition |
| Limitations/Extensions |
State limitations of Bohr’s model |
JEE-Level Examples
In your JEE Mains, you encounter questions of this kind. Prepare them well.
Example 1: Calculate the wavelength of a spectral line in the Balmer series for hydrogen when an electron transitions from
to
.
Solution:
Example 2: Find the charge-to-mass ratio of an electron in a cathode ray tube with electric field , magnetic field , and path radius .
Solution:
NCERT Revision Notes Chemistry
Commonly asked questions
How can the existence of spectra help prove that energy levels in atoms exist?
The existence of atomic spectra tells us that energy levels in atoms are quantised. When atoms absorb or emit light, they do so at specific wavelengths. They lead to line spectra instead of a continuous spectrum. Now, every line corresponds to an electron that transitions between fixed energy levels. This is to make the photon's energy equal to the difference between them. If energy levels were not discrete, the spectra would be continuous. So, the line spectra provide direct evidence that electrons in atoms occupy quantised energy states.
What is the role of Bohr's model in the development of quantum theory?
Bohr’s model played an important role in the development of quantum theory. It introduced the idea of quantised electron orbits. It disproved claims of classical mechanics, which predicted that electrons would spiral into the nucleus. Bohr proposed that electrons can exist only in specific, stable energy levels called stationary states. There would be transitions between these levels that would help Bohr explain the line spectra of hydrogen. That together linked the atomic structure with the concept of energy quantisation. Even though the model could not explain multi-electron atoms, it laid the foundation for modern quantum mechanics.
Chemistry Structure of Atom Exam
Student Forum
Other Topics under this Chapter
- Isotope Meaning
- Mole Concept
- What are Electrons
- Isotopes and Isobars
- What is a Saturated solution
- Difference between Atom and Ion
- Discovery of Sub Atomic Particles
- Atomic Models
- Developments Leading to Bohr's Model of Atom
- Quantum Mechanical Model of Atom
- Bohr's Model of Hydrogen Atom
- Towards Quantum Mechanical Model of Atom
- Structure of Atom
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test