
NCERT Solutions for Class 11 Chemistry Chapter 6 – Equilibrium deals with the dynamic nature of chemical and ionic equilibria in reversible reactions. It offers a comprehensive study material for Class 11 science students. They can master the key topics such as Le Chatelier’s Principle, Law of Mass Action, equilibrium constants, and factors affecting equilibrium.
The solutions are created as per the latest CBSE syllabus. It is ideal for competitive exam preparation, doubt clearance, and strengthening conceptual clarity. Students will also get the link to download the PDF, which offers well-structured answers.
For a deeper understanding of key concepts and quick revision, students should refer to Class 11 Chemistry NCERT Notes. It provides concise summaries and practice questions for each chapter.
- Class 11 Chemistry Structure Equilibrium NCERT Solution PDF: Download PDF for Free
- Important Formulae of Class 11 Chemistry Chapter 6 Equilibrium
- Class 11th Chemistry Equilibrium: Key Topics, Weightage
- Equilibrium Solutions
- Equilibrium Class 11 Chemistry - FAQs
Class 11 Chemistry Structure Equilibrium NCERT Solution PDF: Download PDF for Free
Download PDF from the link below.
Class 11 Chemistry Chapter 1 Some Basic Concepts of Chemistry NCERT Solution PDF: Download Free PDF
Important Formulae of Class 11 Chemistry Chapter 6 Equilibrium
Important Formulae of Chemical Equilibrium for CBSE and Competitive Exams
-
Equilibrium Constant Relations:
- If reaction is reversed:
- If coefficients are multiplied by :
-
Degree of Ionization ( ):
-
pH of Strong Acid/Base:
-
Ka & Kb Relationship with pKa & pKb:
-
Henderson-Hasselbalch Equation for Buffer:
-
Solubility Product ( ) and Solubility ( ):
Class 11th Chemistry Equilibrium: Key Topics, Weightage
It is always a smart way to start any chapter preparation by first knowing all the topics covered. The following are the topics covered in the Class 11 Chemistry Chapter 6 Equilibrium:
| Exercise | Topic | Sub-Topics |
|---|---|---|
| 6.1 | Equilibrium in Physical Processes |
|
| 6.2 | Equilibrium in Chemical Process - Dynamic Equilibrium | - |
| 6.3 | Law of Chemical Equilibrium and Equilibrium Constant | - |
| 6.4 | Homogeneous Equilibria |
|
| 6.5 | Heterogeneous equilibrium | - |
| 6.6 | Application of Equilibrium Constant |
|
| 6.7 | Relationship between Equilibrium Constant K, Reaction Quotient Q and Gibbs Energy G | - |
| 6.8 | Factors Affecting Equilibria |
|
| 6.9 | Ionic Equilibrium in Solution | - |
| 6.10 | Acids, Bases and Salts |
|
| 6.11 | Ionizations of Acids and Bases |
|
| 6.12 | Buffer Solutions |
|
| 6.13 | Solubility Equilibria of Sparingly Soluble Salts |
|
Chapter 6 Equilibrium has almost the same weightage in both the NEET and JEE Mains exams. See below the table to know about the weightage:
| Exam | Number of Questions | Weightage |
|---|---|---|
| NEET | 1-2 questions | 4-8% |
| JEE Main | 1-2 questions | 4-8% |
Get the details of the chapter-wise weightage of the Class 11 Chemistry NCERT solutions and also download each chapter's PDF - Explore Now.
Equilibrium Solutions
| 7.1. A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased. (a) What is the initial effect of the change on the vapour pressure? (b) How do the rates of evaporation and condensation change initially? (c) What happens when equilibrium is restored finally and what will be the final vapour pressure? |
| Answer: (a) On increasing the volume of the container, the vapour pressure will initially decrease because the same amount of vapours is now distributed over a larger space. |
| 7.2. What is Kc for the following equilibrium when the equilibrium concentration of each substance is: [SO2] = 0.60M, [O2] = 0.82M and [SO3] = 1.90 M? 2SO2(g) + O2(g) ⇌ 2SO3(g) |
| Answer: KC= [SO3]2 / [SO2]2[O2] = (1.90)2 / (0.60)2(0.82) = 12.229 L mol-1 |
| 7.3. At a certain temperature and total pressure of 105Pa, iodine vapour contains 40% by volume of I atoms I2 (g) ⇌ 2I (g) Calculate Kp for the equilibrium. |
| Answer: According to the given condition, Total pressure of equilibrium mixture = 105 Pa Partial pressure of iodine atoms (I), PI = 40 % of 105 Pa = 0.4 x 105 Pa Partial pressure of iodine molecules (I2), PI2 = 60 % of 105 Pa = 0.6 x 105 Pa Kp for the equilibrium = (PI)2/ PI2 = (0.4 x 105 Pa)2 / (0.6 x 105 Pa) = 2.67 x 104 Pa |
| 7.4. Write the expression for the equilibrium constant, Kc for each of the following reactions (i) 2NOCl (g) ⇌ 2NO (g) + Cl2 (g) (ii) 2Cu(NO3)2 (s) ⇌ 2CuO (s) + 4NO2 (g) + O2 (g) (iii) CH3COOC2H5 (aq) + H2O (l) ⇌ CH3COOH (aq) + C2H5OH (aq) (iv) Fe3+ (aq) + 3OH– (aq) ⇌ Fe(OH)3(s) (v) I2 (s) + 5F2⇌2IF5 |
| Answer: (i) The expression for the equilibrium constant is Kc= [NO]2[Cl2] / [NOCl]2. |
Commonly asked questions
7.63. Predict if the solutions of the following salts are neutral, acidic or basic:
NaCl, KBr, NaCN, NH4NO3, NaNO2 and
(i) NaCl:
NaCl+H2O↔NaOH+HCl
Strong base Strong acid
Therefore, it is a neutral solution.
(ii) KBr:
KBr+H2O↔KOH+HBr
Strong base Strong acid
Therefore, it is a neutral solution.
(iii) NaCN:
NaCN+H2O↔HCN+NaOH
Weak acid Strong base
Therefore, it is a basic solution.
(iv) NH4NO3
NH4NO3+H2O↔NH4OH+HNO3
Weak base Strong acid
Therefore, it is an acidic solution.
(v) NaNO2
NaNO2+H2O↔NaOH+HNO2
Strong base Weak acid
Therefore, it is a basic solution.
(vi) KF
KF+H2O↔KOH+HF
Strong base Weak acid
Therefore, it is a basic solution.
7.20. One of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and CO2.
FeO (s) + CO (g) Fe (s) + CO2 (g); Kp = 0.265 atm at 1050K
What are the equilibrium partial pressures of CO and CO2 at 1050 K if the initial partial pressures are: pCO= 1.4 atm and pCO2= 0.80 atm?
The initial partial pressures of CO and CO2 are 1.40 atm and 0.8. atm respectively.
The expression for the reaction quotient is:
Qp=PCO2 / PCO=0.80 / 1.4 = 0.571
As the value of the reaction quotient is greater than the value of the equilibrium constant, the reaction will move in the backward direction.
To attain the equilibrium, the partial pressure of CO2 will decrease and the partial pressure of CO will increase.
Let p atm be the decrease in the partial pressure of CO2. The increase in the partial pressure of CO will be p atm.
The equilibrium partial pressure of CO2 is (0.80 – p) atm.
The equilibrium partial pressure of CO is (1.4 + p) atm.
The equilibrium constant is:
Kp = PCO2 / PCO= (0.80p) / (1.4 + p) =0.265
0.265 (1.4+p) = (0.80 – p)
0.371+0.265p = (0.80 – p)
p= 0.339 atm
The equilibrium partial pressure of CO is 1.4+0.339= 1.739.
The equilibrium partial pressure of CO2 is 0.80?0.339= 0.461 atm.
7.58. The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solution. Calculate the concentrations of strontium and hydroxyl ions and the pH of the solution.
Solubility of Sr (OH)2=19.23g/L
The molecular weight of Sr (OH)2 is 87.6 + 2 (17)=121.6
Then, concentration of Sr (OH)2=19.23 /121.63M=0.1581M
Sr (OH)2 (aq)→Sr2+ (aq)+2 (OH−) (aq)
∴ [Sr2+]=0.1581M
[OH−]=2×0.1581M=0.3126M
Now, Kw= [OH−] [H+]
=> [H+] = 10−14 / 0.3126
=> [H+]=3.2×10−14
∴pH= 13.495
7.72 What is the minimum volume of water required to dissolve 1g of calcium sulphate at 298 K? (For calcium sulphate, Ksp is 9.1 x 10−6).
CaSO4 (s)↔Ca2+ (aq)+SO2−4 (aq)
Ksp= [Ca2+] [ SO2−4]
Let the solubility of CaSO4 be s.
[Ca2+] = [ SO2−4] = s
Then, Ksp=s2
9.1×10−6=s2
s =3.02×10−3mol/L
Molecular mass of CaSO4=136g/mol
Solubility of CaSO4 in gram/L= 3.02×10−3×136=0.41g/L
This means that we need 1L of water to dissolve 0.41g of CaSO4.
Therefore, to dissolve 1g of CaSO4 we require = 10.41L= 2.44Lof water.
Assertion and Reason:
Directions:
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
7.74. Assertion: OH– is called the conjugate base of an acid H2O.
Reason: NH4+ is called conjugate acid of the base NH3.
(b) Both are correct statements but R is not the reason for A. The acid-base pair that differs only by one proton is called a conjugate acid-base pair. Therefore, OH– is called the conjugate base of an acid H2O and NH4+ is called conjugate acid of the base NH3. If Brönsted acid is a strong acid then its conjugate base is a weak base and vice-versa. It may be noted that conjugate acid has one extra proton and each conjugate base has one less proton.
7.23. At 1127 K and 1 atm pressure, a gaseous mixture of CO and CO2 in equilibrium with solid carbon has 90.55% CO by mass.
C (s) + CO2 (g) ⇌ 2CO (g)
Calculate Kc for this reaction at the above temperature.
Let the mixture has 100g as total mass.
So, The masses of CO and CO2? are 90.55g and 100 − 90.55 = 9.45 g respectively.
Therefore, te number of moles of CO
n= 90.55 / 28 ?= 3.234.
The number of moles of CO2?
n= 9.45 / 44 ?= 0.215.
The mole fraction of CO
= 3.234? / (3.234+0.215) =0.938.
The mole fraction of CO2?
= 1−0.938=0.062.
The partial pressure of CO is the product of the mole fraction of CO and the total pressure.
It is 0.938×1=0.938 atm.
The partial pressure of carbon dioxide is 0.062×1=0.042 atm.
The expression for the equilibrium constant is:
Kp?=?? (PCO)2 / (PCO2) ??=(0.938)2 / (0.062) ?=14.19
Δng?= 2−1=1
Kc?= Kp?(RT)−Δn=14.19×(0.0821×1127)−1= 0.153.
7.64. The ionization constant of chloroacetic acid is 1.35 × 10–3. What will be the pH of 0.1M acid and its 0.1M sodium salt solution?
Given Ka=1.35 x 10−3
For acid solution:
[H+] = (KaC)1/2 = (0.00135 x 0.1)1/2 = 0.0116M
pH = – log [H+] = –log0.0116 = 1.936
For 0.1M sodium salt solution
ClCH2COONa is the salt of a weak acid i.e., ClCH2COOH and a strong base i.e., NaOH.
pKa = -logKa = log (0.00135) = 2.8697
pKw = 14
logc = log0.1 = −1
pH = 0.5 [pKw + pKa + logc] = 0.5 [14 + 2.8697 -1]
= 7.935
7.65. Ionic product of water at 310 K is 2.7 × 10–14. What is the pH of neutral water at this temperature?
Ionic product,
Kw= [H+] [OH−]
Let [H+]= x
Since [H+]= [OH−], Kw=x2.
⇒Kw at 310K is 2.7×10−14
∴2.7×10−14=x2
⇒x=1.64×10−7
⇒ [H+]=1.64×10−7
⇒ pH= −log [H+]=−log [1.64×10−7]=6.78
Hence, the pH of neutral water is 6.78.
7.56. The pH of milk, black coffee, tomato juice, lemon juice and egg white are 6.8, 5.0, 4.2, 2.2 and 7.8 respectively. Calculate corresponding hydrogen ion concentration in each.
The hydrogen ion concentration in the given substances can be calculated by using the given relation: pH=−log [H+]
Hence, [H+] = 10−pH
Milk: [H+] = 10−6.8 = 1.58×10−7M
Black coffee: [H+] = 10−5.0 =1×10−5M
Tomato juice: [H+] = 10−4.2 =6.31×10−5M
Lemon juice: [H+]=10−2.2 = 6.31×10−3M
Egg white: [H+]=10−7.8=1.58×10−8M
7.71. What is the maximum concentration of equimolar solutions of ferrous sulphate and sodium sulphide so that when mixed in equal volumes, there is no precipitation of iron sulphide?
(For iron sulphide, Ksp=6.3 x 10−18)
Let the maximum concentration of each solution be x mol/L. After mixing, the volume of the concentrations of each solution will be reduced to half i.e., x/2.
∴ [FeSO4]= [Na2S]=x / 2
Then, [Fe2+]= [FeSO4]=x/ 2
Also, [S2−]= [Na2S]=x/2
FeS (x)↔Fe2+ (aq)+S2− (aq)
Ksp= [Fe2+] [S2−]
= >6.3×10−18= (x/2) (x/2)
x2/4=6.3×10−18
⇒x= 5.02×10−9
If the concentrations of both solutions are equal to or less than 5.02×10−9M, then there will be no precipitation of iron sulphide.
7.67. Determine the solubility of silver chromate, barium chromate, ferric hydroxide, lead chloride and mercurous iodide at 298K from their solubility product constants given in Table 7.9 (page 221). Determine also the molarities of individual ions.
1. Silver chromate:
Ag2CrO4→2Ag++CrO42−
Then, [Ag+] = (2s), [CrO42−] = s
Ksp= [Ag+]2 [CrO42−]
s = 0.65×10−4M
So, [Ag+] = 2s = 1.30 x 10−4M, [CrO42−] = 6.5 x 10-5M
2. Barium Chromate:
BaCrO4→Ba2++CrO42−
[Ba2+] = [CrO42−] = s
Then, Ksp= [Ba2+] [CrO42−] = s x s
= > 1.2 x 10-10M = s2
= > s = 1.09 x 10-5M
3. Ferric Hydroxide:
Fe (OH)3→Fe3+ + 3OH−
Then [Fe3+] = s, [OH−] = 3s
Ksp= [Fe3+] [OH−]3
Let s be the solubility of Fe (OH)3
[Fe3+] = s = 1.38 x 10-10M
[OH−] = 3s = 4.14 x 10-10M
4. Lead Chloride:
PbCl2→Pb2++2Cl−
Then, [Pb2+] = s, [Cl−] = 2s
Ksp= [Pb2+] [Cl−]2
= >Ksp=s x (2s)2 =4s3
⇒1.6×10−5=4s3
⇒0.4×10−5=s3
=>4×10−6=s3
[Pb2+] = s=1.58×10−2M
[Cl−] = 2s=3.16×10−2M
5. Mercurous Iodide:
Hg2I2→ 2Hg++2I−
[Hg2+] = [I−] = 2s
Ksp= [Hg2+]2 [I−]2
=>4.5 x 10-29 = (2s)2 (2s)2 = 16s4
=> s = 4.09 x 10-8 M
Therefore, [Hg2+] = [I−] = 2s = 8.18 x 10-8 M
7.68. The solubility product constant of Ag2CrO4 and AgBr are 1.1×10−12and 5.0×10-13 respectively. Calculate the ratio of the molarities of their saturated solutions.
Silver chromate: Ag2CrO4? ? 2Ag+ + CrO42−?
[Ag+] = 2s1? , CrO42−? = s1?
Ksp? = (2s1? )2 (s1? ) = 4s3 = 1.1×10−12
s1? = 6.5 × 10−5 . (1)
Silver bromide: AgBr? Ag+ + Br−
[Ag+] = [Br−] = s2?
Ksp? = (s2? ) × (s2? ) = (s2)2? = 5.0 × 10−13
s2? =7.07×10−7. (2)
Divide equation (1) by equation (2) to obtain the ratio of the molarities of saturated solutions:
? s1/s2? = (6.50×10−5)/ (7.07×10−7)? = 91.9
7.69. Equal volumes of 0.002 M solutions of sodium iodate and cupric chlorate are mixed together. Will it lead to precipitation of copper iodate? (For cupric iodate, Ksp = 7.4 × 10–8).
After mixing, the concentration of NaIO3? is 0.002 / 2? =0.001M.
After mixing, the concentration of Cu (ClO3? )2? is 0.002? / 2 =0.001M.
NaIO3? Na++IO3−?
[IO3−? ]=0.001M
Cu (ClO3? )2? Cu2++2ClO3−?
[Cu2+]=0.001M
The ionic product of Cu (IO3? )2? is
[Cu2+] [I−]2=0.001×0.0012=1×10−9
As the ionic product is less than the solubility product, no precipitation will occur.
7.70. The ionization constant of benzoic acid is 6.46×10−5 and Ksp for silver benzoate is 2.5×10−13. How many times is silver benzoate more soluble in a buffer of pH= 3.19 compared to its solubility in pure water?
Since pH=3.19,
[H3O+]=6.46×10−4M
C6H5COOH+H2O↔C6H5COO−+H3O
Ka= [C6H5COO−] [H3O+] / [C6H5COOH]
[C6H5COOH] / [C6H5COO−]= [H3O+] / Ka=6.46×10−4 / 6.46×10−5=10
Let the solubility of C6H5COOAg be xmol/L.
Then,
[Ag+]=x
[C6H5COOH]+ [C6H5COO−]=x
10 [C6H5COO−]+ [C6H5COO−]=x
[C6H5COO−]=x / 11
Ksp = [Ag+] [C6H5COO−]
= >2.5×10−13=x (x / 11)
= >x=1.66×10−6mol/L
Thus, the solubility of silver benzoate in a pH3.19 solution is 1.66×10−6mol/L.
Now, let the solubility of C6H5COOAg be x′mol/L.
Then, [Ag+]=x′Mand [C6H5COO−]=x′M
Ksp= [Ag+] [C6H5COO−]
Ksp= (x′)2
x′= (Ksp)1/2= (2.5×10−13)1/2 = 5×10−7mol/L
∴x / x′= (1.66×10−6) / (5×10−7) =3.32
Hence, C6H5COOAg is approximately 3.317 times more soluble in a low pH solution.
7.22. Bromine monochloride (BrCl) decomposes into bromine and chlorine and reaches the equilibrium:
2BrCl (g) ⇌ Br2 (g) + Cl2 (g)
The value of Kc is 32 at 500 K. If initially pure BrCl is present at a concentration of 3.3 x10-3mol L-1, what is its molar concentration in the mixture at equilibrium?
Let x moles of BrCl decompose in order to attain the equilibrium. The initial molar concentration and the molar concentration at equilibrium point of different species may be represented as follows:
2BrCl2?(g) ?→?Ar2?(g)+Cl2?(g)
3.3×10−3m 0 0
3.3×10−3−2αα? α
∴ Kc?= (α×α)? /(3.3×10−3−2α)2
⇒ α2 / (3.3×10−3−2α)2?=32
⇒ α / (3.3×10−3−2α)2?=4
⇒ α=18.67×10−3−8 α
⇒ (1+ 8 α =18.67×10−3
⇒ α= 1.5162×10−3
∴ Molar concentration of BrCl at equation
[BrCl] =3.3×10−3−2α
=3.3×10−3−2×1.562×10−3
=0.267×10−3M
7.24. Calculate (a) ∆Gϴ and (b) the equilibrium constant for the formation of NO2 from NO and O2 at 298 K
NO (g) + ½ O2 (g)⇌ NO2 (g)
where
ΔfGϴ (NO2) = 52.0 kJ/mol
ΔfGϴ (NO) = 87.0 kJ/mol
ΔfGϴ (O2) = 0 kJ/mol
(a) ΔG? = Δf?G?(NO2?) − [Δf?G?(NO) + ½ ?Δf?G?(O2?)]
= 52.0 − 87.0 −1/2 × 0
= −35 kJ/mol
(b) logK = − ΔG? / 2.303RT = − 35×103 / (2.303 × 8.314 × 298) ?
= 6.314
K = antilog (6.314)
= 1.362 × 106
7.18. Ethyl acetate is formed by the reaction between ethanol and acetic acid and the equilibrium is represented as:
CH3COOH (l) + C2H5OH (l) ⇌CH3COOC2H5 (l) + H2O (l)
(i) Write the concentration ratio (reaction quotient), Qc, for this reaction (note: water is not in excess and is not a solvent in this reaction)
(ii) At 293 K, if one starts with 1.00 mol of acetic acid and 0.18 mol of ethanol, there is 0.171 mol of ethyl acetate in the final equilibrium mixture. Calculate the equilibrium constant.
(iii) Starting with 0.5 mol of ethanol and 1.0 mol of acetic acid and maintaining it at 293 K, 0.214 mol of ethyl acetate is found after sometime. Has equilibrium been reached?
(i) The concentration ratio (Concentration quotient), Qc for the reaction is:
Qc = [CH3COOC2H5] [ H2O] / [CH3COOH] [C2H5OH]
(ii)
| CH3COOH | C2H5OH | CH3COOC2H5 | H2O |
Initial molar concentration | 1.0 mol | 0.18 mol | 0 | 0 |
Molar concentration at equilibrium | (1 – 0.171) = 0.829 mol | (0.18 – 0.171) = 0.009 mol | 0.171 mol | 0.171 mol |
Applying
Kc = [CH3COOC2H5] [H2O] / [CH3COOH] [C2H5OH]
= (0.171 mol) x (0.171 mol) / (0.829 mol) (0.009 mol)
= 3.92
(iii)
| CH3COOH | C2H5OH | CH3COOC2H5 | H2O |
Initial molar concentration | 1.0 mol | 0.5 mol | 0.214 mol | 0.214 mol |
Molar concentration at equilibrium | (1 – 0.214) = 0.786 mol | (0.5 – 0.214) = 0.286 mol | 0.214 mol | 0.214 mol
|
Qc = [CH3COOC2H5] [H2O] / [CH3COOH] [C2H5OH]
= (0.214 mol) x (0.214 mol) / (0.786 mol) (0.286 mol)
= 0.204
Since Qc is less than Kc, this means that the equilibrium has not been reached. The reactants are still taking part in the reaction to form the products.
7.62. A 0.02M solution of pyridinium hydrochloride has pH = 3.44. Calculate the ionization constant of pyridine.
pH=3.44
We know that,
pH=−log [H+]
∴ [H+]=3.63×10−4
Then, Kb= (3.63×10−4)2 / 0.02 (? concentration =0.02M)
⇒Kb=6.6×10−6
Now, Kb=Kw / Ka
⇒Ka=Kw / Kb=10−14 / 6.6×10−6=1.51×10−9
7.19. A sample of pure PCl5 was introduced into an evacuated vessel at 473 K. After equilibrium was reached, the concentration of PCl5 was found to be 0.5 x 10-1 mol L-1. If Kc is 8.3 x 10-3 what are the concentrations of PCl3 and Cl2 at equilibrium?
Let the initial molar concentration of PCl5 per litre = x mol
Molar concentration of PCl5 at equilibrium = 0.05 mol
Therefore, moles of PCl5 decomposed = (x – 0.05) mol
Moles of PCl3 formed = (x – 0.05) mol
Moles of Cl2 formed = (x – 0.05) mol
The molar conc./litre of reactants and products before the reaction and at the equilibrium point are:
| PCl5 | PCl3 | Cl2 |
Initial moles/litre | x | 0 | 0 |
At equilibrium | 0.05 | x – 0.05 | x – 0.05 |
Kc = [PCl3] [Cl2] / [PCl5]
=> 8.3 x 10-3 = (x – 0.05)2 / 0.05
=> (x – 0.05) = (4.15 x 10-4)1/2 = 2.037 x 10-2 = 0.02 moles
=> x = 0.05 + 0.02 = 0.07 mol
Therefore, at equilibrium:
Moles of PCl3 formed = (x – 0.05) mol = 0.07 – 0.05 = 0.02 mol
Moles of Cl2 formed = (x – 0.05) mol = 0.07 – 0.05 = 0.02 mol
7.25. Does the number of moles of reaction products increase, decrease or remain same when each of the following equilibria is subjected to a decrease in pressure by increasing the volume?
(a) PCl5 (g) ⇌ PCl3 (g) + Cl2 (g)
(b) CaO (s) + CO2 (g) ⇌ CaCO3 (s)
(c) 3Fe (s) + 4H2O (g) ⇌ Fe3O4 (s) + 4H2 (g)
(i) Pressure will increase in the forward reaction and number of moles of products will increase.
(ii) Pressure will increase in backward reaction and number of moles of products will decrease.
(iii) The change in pressure will have no effect on the equilibrium constant and there will be no change in the number of moles.
7.26. Which of the following reactions will get affected by increasing the pressure? Also mention whether the change will cause the reaction to go into forward or backward direction.
(i) COCl2 (g) ⇌ CO (g) + Cl2 (g)
(ii) CH4 (g) + 2S2 (g) ⇌ CS2 (g) + 2H2S (g)
(iii) CO2 (g) + C (s) ⇌ 2CO (g)
(iv) 2H2 (g) + CO (g) ⇌ CH3OH (g)
(v) CaCO3 (s) ⇌CaO (s) + CO2 (g)
(vi) 4NH3 (g) + 5O2 (g) ⇌ 4NO (g) + 6H2O (g)
Only those reactions will be affected by increasing the pressure in which the number of moles of the gaseous reactants and products are different (i.e. when np ≠ nr) (gaseous). In general,
- The reaction will go to the left if np> nr.
- The reaction will go to the right if nr> np.
Keeping this in mind,
(i) Increase in pressure will favour backward reaction because np (2) > nr (1)
(ii) Increase in pressure will not affect equilibrium because np = nr = 3.
(iii) Increase in pressure will favour backward reaction because np (2) > nr (1)
(iv) Increase in pressure will favour forward reaction because np (1) < nr (2)
(v) Increase in pressure will favour backward reaction because np (1) > nr (0).
(vi) Increase in pressure will favour backward reaction because np (10) > nr (9).
7.27. The equilibrium constant for the following reaction is 1.6 ×105 at 1024K
H2(g) + Br2(g) ⇌ 2HBr(g)
Find the equilibrium pressure of all gases if 10.0 bar of HBr is introduced into a sealed container at 1024K.
For the equilibrium reaction
2HBr (g) ? H2(g) + Br2(g) , the equilibrium constant is K = 1/1.6×105.
Initial pressure 10 0 0
At equilibrium 10-p p/2 p/2
The equilibrium constant expression is
Kp=PH2PBr2 / (PHBr)2=(p/2)×(p/2) / (10−p)2=1 / 1.6×105
p2 / 4(10−p)2=1 / 1.6×105
400p= 20−2p
p= 0.0498 bar
The equilibrium pressures are
PH2=PBr2=0.0498 / 2=0.0249 bar
PHBr=10−0.0498=10 bar
7.59. The ionization constant of propanoic acid is 1.32 × 10–5. Calculate the degree of ionization of the acid in its 0.05M solution and also its pH. What will be its degree of ionization if the solution is 0.01M in HCl also?
Let the degree of ionization of propanoic acid be α. Then, representing propionic acid as HA, we have:
HA + H2O ⇔ H3O+ + A−
(.05−0.0α)≈0.5 0.05α 0.05α
Ka= [H3O+] [A−] / [HA]
= (0.05α) (0.05α) / 0.05
= 0.05α2
=> α = (Ka /0.05)1/2
=1.63×10−2
Then, [H3O+]=.05α=.05×1.63×10−2= Kb.15×10−4M
∴pH=3.09
In the presence of 0.1M of HCl, let α' be the degree of ionization.
Then, [H3O+]=0.01
[A−]= 005α′
[HA]=.05
Ka= (0.01×.05α′) /0.05
=>1.32×10−5=.01×α′
=>α′=1.32×10−3
7.60. The pHof 0.1M of cyanic acid (HCNO) is 2.34. Calculate the ionization constant of the acid and its degree of ionization in the solution.
c=0.1M
pH= −log [H+]
=> 2.34 = −log [H+]
So, −log [H+]= 2.34
=> [H+]= 4.5×10−3
Also,
[H+]= cα
=>4.5×10−3= 0.1×α
=> α=4.5×10−2= 0.045
Then,
Ka = α2/c = (45×10−3)2/ 0.1
=202.5×10−6
=2.02×10−4
7.61. The ionization constant of nitrous acid is 4.5×10−4. Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.
Since NaNO2 is the salt of a strong base (NaOH) and a weak acid (HNO2);
NO2− + H2O ↔ HNO2 + OH−
Then, Kb= [HNO2] [OH−] / [NO2−]
⇒ Kw/ Ka = (10−14) / (4.5×10−4)
= 0.22×10−10
Now, if x moles of the salt undergo hydrolysis, and then the concentration of various species present in the solution will be:
[NO2−]= 0.04−x; 0.04
[HNO2]= x
[OH−]= x
Kb= x2 / 0.04
= 0.22×10−10
x2= 0.0088×10−10
x= 0.093×10−5
∴ [OH−]= 0.093×10−5 M
[H3O+]=10−14 / 0.093×10−5=10.75×10−9 M
⇒ pH= −log (10.75×10−9)=7.96
Now, degree of hydrolysis
= x / 0.04= (0.093×10−5)/ 0.04
= 2.325×10−5
7.93. (i) Define Le Chatelier’s principle.
(ii) Following reactions occur in a Blast furnace.
Fe2O3(s) + 3CO(g) ———–>2Fe(s) + 3CO2(g)
Use Le Chatelier’s principle to predict the direction of reaction when equilibrium mixture is disturbed by
(a) adding Fe2O3 (b) removing CO2.
(c) removing CO.
(i) Le Chatelier's principle states that the change in any factor such as temperature, pressure, concentration, etc. will cause the equilibrium to shift in such a direction so as to reduce or counteract the effect of the change.
(ii) (a) On adding Fe2O3 (s), the equilibrium will remain unaffected.
(b) By removing CO2 (g), the equilibrium will be shifted in the forward direction.
(c) By removing CO (g), the equilibrium will be shifted in the backward direction.
7.3. At a certain temperature and total pressure of 105Pa, iodine vapour contains 40% by volume of I atoms
I2 (g) ?? 2I (g)
Calculate Kp for the equilibrium.
According to the given condition,
Total pressure of equilibrium mixture = 105 Pa
Partial pressure of iodine atoms (I), PI = 40 % of 105 Pa
= 0.4 x 105 Pa
Partial pressure of iodine molecules (I2), PI2 = 60 % of 105 Pa
= 0.6 x 105 Pa
Kp for the equilibrium = (PI)2/ PI2 = (0.4 x 105 Pa)2 / (0.6 x 105 Pa)
= 2.67 x 104 Pa
7.1. A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
(a) What is the initial effect of the change on the vapour pressure?
(b) How do the rates of evaporation and condensation change initially?
(c) What happens when equilibrium is restored finally and what will be the final vapour pressure?
(a) On increasing the volume of the container, the vapour pressure will initially decrease because the same amount of vapours is now distributed over a larger space.
(b) On increasing the volume of the container, the rate of evaporation will increase initially because now more space is available. Since the amount of the vapours per unit volume decrease on increasing the volume, therefore, the rate of condensation will decrease initially.
(c) Finally, equilibrium will be restored when the rates of the forward and backward processes become equal. However, the vapour pressure will remain unchanged because it depends upon the temperature and not upon the volume of the container.
7.2. What is Kc for the following equilibrium when the equilibrium concentration of each substance is: [SO2] = 0.60M, [O2] = 0.82M and [SO3] = 1.90 M?
2SO2(g) + O2(g) ?? 2SO3(g)
KC= [SO3]2 / [SO2]2 [O2]
= (1.90)2 / (0.60)2 (0.82)
= 12.229 L mol-1
7.4. Write the expression for the equilibrium constant, Kc for each of the following reactions
(i) 2NOCl (g) ?? 2NO (g) + Cl2 (g)
(ii) 2Cu(NO3)2 (s) ?? 2CuO (s) + 4NO2 (g) + O2 (g)
(iii) CH3COOC2H5 (aq) + H2O (l) ?? CH3COOH (aq) + C2H5OH (aq)
(iv) Fe3+ (aq) + 3OH– (aq) ?? Fe(OH)3(s)
(v) I2 (s) + 5F2??2IF5
(i) The expression for the equilibrium constant is Kc= [NO]2 [Cl2] / [NOCl]2.
(ii) The expression for the equilibrium constant is Kc= [NO2]4 [O2] / [ (2Cu (NO3)2]2 = [NO2]4 [O2].
(iii) The expression for the equilibrium constant is Kc= [CH3COOH] [C2H5OH] / [CH3COOC2H5].
(iv) The expression for the equilibrium constant is Kc= 1 / [Fe3+] [OH]3.
(v) The expression for the equilibrium constant is Kc= [IF5]2 / [F2]5.
7.5. Find out the value of Kc for each of the following equilibria from the value of Kp:
(i) 2NOCl (g) ⇌ 2NO (g) + Cl2 (g); Kp= 1.8 × 10–2 at 500 K
(ii) CaCO3 (s) ⇌ CaO (s) + CO2(g); Kp= 167 at 1073 K
Using the equation Kp = Kc (RT)ng
(i) From the given equation, Δng = 3 – 2 = 1,
R = 0.0821 L atm K-1 mol-1
T = 500 K, Kp= 1.8 × 10–2
Thus, Kc = Kp / (RT)ng= (1.8 x 10-2) / (0.0821 L atm K-1 mol-1 x 500 K)
4.4 x 10-4 mol L-1
(ii) From the given equation, Δng =1,
R = 0.0821 L atm K-1 mol-1
T = 1073 K, Kp= 167 atm
Thus, Kc = Kp / (RT)ng= (167 atm) / (0.0821 L atm K-1 mol-1 x 1073 K)
= 1.9 mol L-1
7.6. For the following equilibrium, K =6.3 x 1014 at 1000 K.
NO (g) + O3 —–> NO2 (g) + O2(g).
Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is Kc for the reverse reaction?
For reverse reaction, KC (reverse) = 1/ KC = 1 / 6.3 x 1014 = 1.59 x 10-15
7.7 Explain why pure liquids and solids can be ignored while writing the equilibrium constant expression?
This is because molar concentration of a pure solid or liquid is independent of the amount present.
Since density as well as molar mass of pure liquid or solid is fixed; their molar concentrations are constant.
The concentration of solid or liquid is = = =
At constant temperature, the density and molar mass of pure solid and liquid are constant.
Due to this, their molar concentrations are constant and are not included in the equilibrium constant.
7.8. Reaction between nitrogen and oxygen takes place as follows:
2N2?(g) + O2?(g) ? 2N2?O(g)
If a mixture of 0.482 mol of N2 and 0.933 mol of O2 is placed in a reaction vessel of volume 10 L and allowed to form N2O at a temperature for which Kc = 2.0 x 10-37, determine the composition of the equilibrium mixture.
Let x moles of N2 (g) take part in the reaction. According to the equation, x/2 moles of O2 (g) will react to form x moles of N2O (g). The molar concentration per litre of different species before the reaction and at the equilibrium point is:
| [N2? ] | [O2] | [N2? O] |
Initial concentration | 0.482? / 10 | 0.933/ 10 | 0 |
Conc. at equilibrium | (0.482 – x)? / 10 | (0.933 – x/2) / 10 | x/ 10 |
The value of equilibrium constant (2.0 x 10-37) is extremely small. This means that only small amounts of reactants have reacted. Therefore, value of x is extremely small and can be omitted as far as the reactants are concerned.
Applying Law of chemical equilibrium, Kc = [N2O]2 / [N2]2 [O2]
= Kc = 2.0 x 10-37
= (x/10)2 / [ (0.482/ 10)2 x (0.933 / 10)2]
= 0.01 x2 / (2.1676 x 10-4)
x2= 43.352 x 10-40
x = 6.6 x 10-20
Therefore, [N2] = 0.0482 mol/L
[O2] = 0.0933 mol/L
[N2O] = 0.1 x x= 0.1 x 6.6 x 10-20 = = 6.6 x 10-21 mol/L
7.9. Nitric oxide reacts with Br2and gives nitrosyl bromide as per reaction given below:
2NO (g) + Br2 (g) ?? 2NOBr (g)
When 0.087 mole of NO and 0.0437 mole of Br2 are mixed in a closed container at constant temperature, 0.0518 mole of NOBr is obtained at equilibrium. Determine the compositions of the equilibrium mixture.
According to the equation, 2 moles of NO (g) react with 1 mole of Br2 (g) to form 2 moles of NOBr (g). The composition of the equilibrium mixture can be calculated as follows:
No. of moles of NOBr (g) formed at equilibrium = 0.0518 mol
No. of moles of NO (g) taking part in reaction = 0.0518 mol
No. of moles of NO (g) left at equilibrium = 0.087 – 0.0518 = 0.0352 mol
No. of moles of Br2 (g) taking part in reaction = 1/2 x 0.0518 = 0.0259 mol
No. of moles of Br2 (g) left at equilibrium = 0.0437 – 0.0259 = 0.0178 mol
The initial molar concentration and equilibrium molar concentration of different species may be represented as:
2NO (g) + Br2 (g) ——————> 2NOBr (g)
| NO | Br2 | NOBr |
Initial moles | 0.087 | 0.0437 | 0 |
Change | -2x | -x | 2x |
Moles at equilibrium | 0.0352 | 0.0178 | 0.0518 |
7.10. At 450 K, Kp = 2.0 × 1010/bar for the given reaction at equilibrium.
2SO2(g) + O2(g) ?? 2SO3 (g)
What is Kc at this temperature?
Kp = Kc (RT)? ng
=> Kc = Kp (RT)-? ng
Putting the values of Kp= 2.0x1010 bar-1, R= 0.083 L bar K-1 mol-1, T = 450 K, and? ng = 2-3= -1
=> Kc = (2.0 x 1010 bar-1) x [ (0.083 L bar K-1 mol-1) x (450 K)]- (-1)
= 7.47 x 1011 mol-1 L
= 7.47 x 1011 M-1
7.11. A sample of HI (g) is placed in a flask at a pressure of 0.2 atm. At equilibrium partial pressure of HI (g) is 0.04 atm. What is Kp for the given equilibrium?
2HI (g) ?? H2 (g) + I2 (g)
pHI = 0.04 atm, pH2 = 0.08 atm, pI2 = 0.08 atm
Kp= (pH2 x pI2) / (pHI)2 = (0.08 x 0.08) / (0.04 x 0.04)
= 4.0
7.12. A mixture of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant Kc for the reaction
N2?(g) + 3H2? (g) ? 2NH3? (g) is 1.7×102
Is this reaction at equilibrium? If not, what is the direction of net reaction?
According to the given equation, concentration quotient,
Qc = [NH3]2 / [N2] [ H2]3
= (8.13/20 mol L-1)2 / (1.57 / 20 mol L-1) x (1.92 / 20 mol L-1)3
= 2.38 x 103
The equilibrium constant (Kc) for the reaction = 1.7 x 10-2
As Qc ≠ Kc, the reaction is not in a state of equilibrium.
7.13. The equilibrium constant expression for a gas reaction is,
Write the balanced chemical equation corresponding to this expression.
Balanced chemical equation for the reaction is
4 NO (g) + 6 H2O (g)? 4 NH3 (g) + 5 O2 (g)
7.14. One mole of H2O and one mole of CO are taken in a 10 litre vessel and heated to 725 K, at equilibrium point 40 percent of water (by mass) reacts with carbon monoxide according to equation.
H2O (g) + CO (g) ? H2 (g) + CO2 (g)
Calculate the equilibrium constant for the reaction.
Number of moles of water originally present = 1 mol
Percentage of water reacted= 40%
Number of moles of water reacted= 1 x 40/100 = 0.4 mol
Number of moles of water left= (1 – 0.4) = 0.6 mole
According to the equation, 0.4 mole of water will react with 0.4 mole of carbon monoxide to form 0.4 mole of hydrogen and 0.4 mole of carbon dioxide.
Thus, the molar conc., per litre of the reactants and products before the reaction and at the equilibrium point are as follows:
| H2O | CO | H2 | CO2 |
Initial moles / litre | 1/10 | 1/10 | 0 | 0 |
At Equilibrium | (1 – 0.4) / 10 = 0.6/10 | (1 – 0.4) / 10 = 0.6/10 | 0.4/10 | 0.4/10
|
Equilibrium constant, Kc= [H2] [CO2] / [H2O] [CO]
= [ (0.4/10) x (0.4/10)] / [ (0.6/10) x (0.6/10)]
= 0.16 / 0.36 = 0.44
7.15. At 700 K, equilibrium constant for the reaction:
H2?(g) + I2 (g) ?2HI (g)
is 54.8. If 0.5 mol L–1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2?(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700K?
H2 (g) + I2 (g)? 2HI (g); K=64, T=700K
2HI? H2 + I2 K=1/64700K
a 0 0
a (1−α) aα/2 aα/2
0.5 x x
x2 / (0.5)2 = 1 / 54.8
x2 = 0.25 / 54.8
x = = 0.068 M
At equilibrium, [H2] = [I2] = 0.068 M
7.16. What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M?
2ICl (g) ⇌ I2 (g) + Cl2 (g); Kc = 0.14
Suppose at equilibrium, the molar concentration of both I2 and Cl2 is x mol L-1.
Kc = [I2] [ Cl2] / [ICl]2= x2 / (0.78 – 2x)2
=>x/ (0.78 – 2x) = (0.14)1/2 = 0.374
=> x= 0.167
[ICl] = (0.78 – 2 x 0.167) = (0.78 – 0.334) = 0.446 M
[I2] = 0.167 M,
[Cl2] = 0.167 M
7.17. Kp =0.04 atm at 898 K for the equilibrium shown below. What is the equilibrium concentration ok C2H6 when it is placed in a flask at 4 atm pressure,and allowed to come to equilibrium.
C2H6(g) ⇌ C2H4(g) + H2(g).
The equilibrium reaction is
C2?H6?(g) ? C2?H4?(g)+H2?(g).
Initial | 4 | 0 | 0 |
Change | −x | x | x |
Equilibrium | 4−x | x | x |
The expression for the equilibrium constant is Kp?= (??PC2?H4??) (PH2) / PC2?H6???.
Substituting the values in the above equation, we get
0.04=x2 / (4−x)
? or x=0.38
Thus, the pressure of ethane is, PC2?H6??=3.62atm.
7.21 Equilibrium constant, Kc for the reaction
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) at 500 Kc is 0.061
At a particular time, the analysis shows that composition of the reaction mixture is 3.0 mol L–1 N2, 2.0 mol L–1 H2 and 0.5 mol L–1 NH3. Is the reaction at equilibrium? If not in which direction does the reaction tend to proceed to reach equilibrium?
According to available data:
N2 = [3.0], H2 = [2.0], NH3 = [0.50]
Qc = [NH3]2 / [N2] [ H2]3
= [0.50]2 / [3.0] [ 2.0]3
= 0.25/24
= 0.0104.
Since the value of Qc is less than that of Kc (0.061), the reaction is not in a state of equilibrium. It will proceed in the forward direction till Qc becomes the same as Kc.
7.28 Dihydrogen gas is obtained from natural gas by partial oxidation with steam as
per following endothermic reaction:
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
(a) Write as expression for Kp for the above reaction.
(b) How will the values of Kp and composition of equilibrium mixture be affected by
(i) Increasing the pressure
(ii) Increasing the temperature
(iii) using a catalyst?
(a) The expression for Kp for the reaction is:
Kp = (pCO) x (pH2) / (pCH4) x (pH2O)
(b) (i) By increasing the pressure, the number of moles per unit volume will increase. In order to decrease the same, the equilibrium gets shifted to the left or in the backward direction. As a result, more of reactants will be formed and the value of Kp will decrease.
(ii) If the temperature is increased, according to Le Chatelier's principle, the forward reaction will be favoured as it is endothermic. Therefore, the equilibrium gets shifted to the right and the value of Kp will increase.
(iii) The addition of catalyst will not change the equilibrium since it influences both the forward and the backward reactions to the same extent. But it will be attained more quickly.
7.29. Describe the effect of:
(i) Addition of H2 (ii) Addition of CH3OH
(iii) Removal of CO (iv) Removal of CH3OH
on the equilibrium of the reaction:
2H2(g) + CO (g) ⇌ CH3OH (g)
(i) Equilibrium will be shifted in the forward direction.
(ii) Equilibrium will be shifted in the backward direction.
(iii) Equilibrium will be shifted in the backward direction.
(iv) Equilibrium will be shifted in the forward direction.
7.30. At 473 K, the equilibrium constant Kc for the decomposition of phosphorus pentachloride (PCl5) is 8.3 x 10-3. If decomposition proceeds as:
PCl5 (g) ⇌ PCl3 (g) + Cl2 (g) ΔrHϴ = 124.0 kJ mol–1
(a) Write an expression for Kc for the reaction
(b) What is the value of Kc for the reverse reaction at the same temperature.
(c) What would be the effect on Kc if
(i) More of PCl5is added (ii) pressure is increased (iii) the temperature is increased.
(a) The equilibrium constant expression is
Kc = [PCl3] [Cl2] / [PCl5]
(b) The equilibrium constant expression for the reverse reaction is
Kc (reverse) = 1/ (8.3×103) =120.48
(c) (i) By adding more of PCl5, value of Kc will remain constant because there is no change in temperature.
(ii) When pressure is increased, the value of the equilibrium constant remains unaffected.
(iii) By increasing the temperature, the forward reaction will be favoured since it is endothermic in nature. Therefore, the value of equilibrium constant will increase.
7.31. Dihydrogen gas used in Haber’s process is produced by reacting methane from natural gas with high temperature steam. The first stage of two stage reaction involves the formation of CO and H2 In second stage, CO formed in first stage is reacted with more steam in water gas shift reaction.
CO (g) + H2O(g) ⇌ CO2(g) + H2(g)
If a reaction vessel at 400°C is charged with an equimolar mixture of CO and steam so that PCO = PH2O = 4.0 bar, what will be the partial pressure of H2 at equilibrium? Kp = 10.1 at 400°C.
Let the partial pressures of CO and H2 be p.
CO (g) + H2?O(g) ? CO2?(g) + H2?(g)
Initial conc. 4 bar 4 bar 0 0
At equilibrium 4-p 4-p p p
The expression for the equilibrium constant is
Kp? = ?PCO2 ??PH2??? / PCO?PH2?O
= (p×p) / (4−p)(4−p) ?= 10.1
p = 12.712- 3.17p
p = 3.04 bar.
7.32. Predict which of the following will have appreciable concentration of reactants and products:
a) Cl2 (g) ⇌2Cl (g) Kc = 5 ×10–39
b) Cl2 (g) + 2NO (g) ⇌2NOCl (g) Kc = 3.7 × 108
c) Cl2 (g) + 2NO2 (g) ⇌2NO2Cl (g) Kc = 1.8
Following conclusions can be drawn from the values of Kc .
(a) Since the value of Kc is very small, this means that the molar concentration of the products is very small as compared to that of the reactants.
(b) Since the value of Kc is quite large, this means that the molar concentration of the products is very large as compared to that of the reactants.
(c) Since the value of Kc is 1.8, this means that both the products and reactants have appreciable concentration.
7.33. The value of Kc for the reaction 3O2(g) ⇌2O3(g) is 2.0 x 10-50 at 25°C. If equilibrium concentration of O2 in air at 25°C is 1.6 x 10-2, what is the concentration of O3?
The equilibrium constant expression is
Kc = [O3]2 / [O2]3
2.0×1050 = [O3]2 / (1.6×102)3
[O3]2=2.0×1050× (1.6×102)3
=8.192×1056
Hence, the equilibrium concentration of ozone is
[O3]= 2.86×1028M
7.34. The reaction, CO(g) + 3H2(g) ⇌ CH4(g) + H2O(g) is at equilibrium at 1300 K in a 1L flask. It also contains 0.30 mol of CO, 0.10 mol of H2 and 0.02 mol of H2O and an unknown amount of CH4 in the flask. Determine the concentration of CH4 in the mixture. The equilibrium constant, Kc for the reaction at the given temperature is 3.90.
Kc = [CH4] [H2O] / [CO] [H2]3
3.90 = [CH4]×0.02 / (0.30) (0.10)3
[CH4] = 3.90×0.30× (0.10)3 / 0.02
=5.85×102 M
Thus, the concentration of methane in the mixture is 5.85×102 M.
7.35. What is meant by conjugate acid-base pair? Find the conjugate acid/base for the following species: HNO2, CN–, HClO4F–, OH–, CO32-, and S2-.
An acid-base pair which differs by a proton only is known as conjugate acid-base pair. For example, HCl, and Cl represents conjugate acid base pair.
The conjugate acid/base for the species HNO2, CN–, HClO4F–, OH–, CO32-, and S2- are NO2–, HCN, ClO4–, HF, H2O, HCO3– and HS–.respectively.
7.36. Which of the following are Lewis Acids?
H2O,BF3, H+ and NH4+
BF3, H+ ions are Lewis acids.
7.37. What will be the conjugate bases for the Bronsted acids: HF, H2SO4 and H2CO3?
Conjugate bases: F–, HSO–4 , HCO–3.
7.38. Write the conjugate acids for the following Bronsted bases:
NH2, NH3 and HCOO–
NH3, NH4+ and HCOOH
7.40. Classify the following species into Lewis acids and Lewis bases and show how these can act as Lewis acid/Lewis base?
(a) OH– (b) F– (c) H+ (d) BCl3
(a) OH– ions can donate an electron pair and act as Lewis base.
(b) F– ions can donate an electron pair and actas Lewis base.
(c) H+ ions can accept an electron pair and act as Lewis acid.
(d) BCl3 can accept an electron pair since Boron atom is electron deficient. It is a Lewis acid.
7.41. The concentration of hydrogen ions in a sample of soft drink is 3.8 x 10-3 M. What is the pH value?
pH = – log [H+] = – log (3.8 x 10-3) = – log 3.8 + 3 = 3 – 0.5798 = 2.4202 = 2.42
7.42. The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
pH = – log [H+] or log [H+] = – pH = – 3.76 = 4.24
Therefore, [H+] = Antilog 4.24 = 1.738 x 10-4 = 1.74 x 10-4 M
7.46. The ionization constant of acetic acid is 1.74 x 10-5. Calculate the degree of dissociation of acetic acid in its 0.05 M solution. Calculate the concentration of acetate ion in the solution and its pH.
The dissociation equilibrium is
CH3? COOH? CH3? COO− + H+.
Let α be the degree of dissociation.
The equilibrium concentrations of CH3? COOH, CH3COO− and H+ are c (1−α), c (α) and c (α) respectively.
The equilibrium constant expression is Kc? = [CH3? COO−] [H+]? / [CH3? COOH].
Kc? = (cα) (cα) / c (1−α)? ≈cα2
α= (Ka? / c)1/2? = (1.74×10−5 / 0.05)1/2?
=1.865×10−2
[CH3? CO−]= [H+]= cα= 0.05×1.865×10−2= 9.33×10−4M
pH= −log [H+]= −log (9.33×10−4)= 3.03
The concentration of acetate ion and its pH are 9.33×10−4 and 3.03 respectively.
7.47. It has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionization constant of the acid and its PKa.
pH= −log [H+]=4.15
[H+]= antilog (−4.15)= 7.08×10−5
[A−]= [H+]=7.08×10−5
The concentration of undissociated acid is 0.01−0.000071=0.009929M.
HA+H2? O? H3? O++A−
Ka? = [H3? O+] [A−] / [HA]? = (7.08×10−5) (7.08×10−5)? / 0.009929
= 5.05×10−7
pKa? = −logKa? = −log5.05×10−7 ≈ 6.3
7.48. Assuming complete dissociation, calculate the pH of the following solutions:
(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH.
(a) 0.003 M HCl
[H3? O+] = [HCl] = 0.003M
pH = −log [H+] = −log (3.0×10−3) = 2.523
(b) 0.005 M NaOH
[OH−] = [NaOH] = 0.005M
[H+] = Kw? / [OH−]? = 10−14/ 0.005? =2×10−12
pH= −log [H+]=−log (2×10−12)=11.699
(c) 0.002M HBr
[H+]= [HBr]=0.002
pH= −log [H+]=−log0.002=2.699
(d) 0.002M KOH
[OH−]= [KOH]=0.002M
[H+]= Kw / [OH−]? =10−14 / 0.002? =5×10−12
pH= −log [H+]=−log (5×10−12)=11.301
7.49. Calculate the pH of the following solutions:
(a) 2g ofTlOH dissolved in water to give 2 litre of the solution
(b) 0.3 g of Ca(OH)2 dissolved in water to give 500 mL of the solution
(c) 0.3 g of NaOH dissolved in water to give 200 mL of the solution
(d) l mL of 13.6 M HCl is diluted with water to give 1 litre of the solution.
(a) For 2g of TlOH dissolved in water to give 2 L of solution:
[TlOH] = [OH−] = (2×1)? / (2×221) = (1 / 221)? M
pOH = −log [OH]− = −log (1/221)?
= 2.35
pH = 14 – pOH = 14 − 2.35 = 11.65
(b) For 0.3 g of Ca (OH)2? dissolved in water to give 500 mL of solution:
[OH−] = 2 [Ca (OH)2? ] = 2 (0.3×1000/500? ) = 1.2M
pOH = −log [OH−] = −log1.2 = 1.79
pH= 14−pOH=14−1.79
=12.21
(c) For 0.3 g of NaOH dissolved in water to give 200 mL of solution:
[OH−]= [NaOH] = 0.3×1000/200? = 1.5M
pOH= −log [OH−] = −log1.5 = 1.43
pH= 14 – pOH = 14 − 1.43
= 12.57
(d) For 1mL of 13.6 M HCl diluted with water to give 1 L of solution:
The molarity of HCl solution after dilution is (13.6×1)/ 1000= 0.0136M.
It is equal to hydrogen ion concentration.
pH = −log [H+]= −log0.0136
= 1.87
7.50. The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the PKa of bromoacetic acid.
H+] = cα = 0.1 × 0.132 = 0.0132M
pH = −log [H+] = −log0.0132
= 1.88
The acid dissociation constant is
Ka? = cα2? / (1−α) = 0.1 × (0.132)2 / (1−0.132)
= 2.01×10−3.
pKa? = −logKa? = −log (2.01×10−3) ≈ 2.7
7.51. The pH of 0.005 M codeine (C18H21N03) solution is 9.95. Calculate the ionization constant and PKb.
pH = 9.95,
pOH = 14 – pH = 14 − 9.95 = 4.05
[OH−] = 10−pOH = 10−4.05 = 8.913 × 10−5
Codeine + H2? O? CodeineH+ + OH−
The ionization constant, Kb? = [CodeineH+] [OH−] / [codeine]?
= [ (8.913×10−5)× (8.913×10−5)] / 5×10−3
= 1.588×10−6.
pKb? = −log (1.588×10−6)
= 5.8
7.52. What is the pH of 0.001 M aniline solution? The ionization constant of aniline can be taken from Table 7.7. Calculate the degree of ionization of aniline in the solution. Also calculate the ionization constant of the conjugate acid of aniline.
Let c be the initial concentration of C6H5NH3+ and x be the degree of ionisation.
C6H5NH2 + H2O? C6H5NH3+ + OH-
c (1-x) cx cx
Kb = [C6H5NH3+] [ OH-] / [C6H5NH2]
= [cx] [cx] / [c (1 – x)]
Since x is very small and negligible 1 – x≈ 1
∴Kb= [cx] [cx] / [c] = cx2
=> x =
=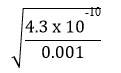
= 6.56 x 10-4
∴ [OH-] = cx = 0.001 x 6.56 x 10-4 = 6.56 x 10-7 M
[H+]= Kw / [OH-] = 10-14 / 6.56 x 10-7 = 1.52 x 10-8
pH= –log [H+] = –log1.52 x 10-8 = 7.818
∴ Ionization constant of the conjugate acid of aniline,
Ka = Kw / Kb
= 10-14 / (4.3 x 10-10)
= 2.32 x 10-5
7.53. Calculate the degree of ionization of 0.05M acetic acid if its pKa value is 4.74.
How is the degree of dissociation affected when its solution also contains
(a) 0.01M (b) 0.1M in HCl?
pKa? =? logKa= 4.74
Ka? = 10? pKa =10?4.74 = 1.8*10?5
Let x be the degree of dissociation. The concentration of acetic acid solution, C = 0.05 M
The degree of dissociation,
x= (Ka / C)1/2? = (1.8*10?5 / 0.05)1/2 ? = 0.019
(a) The solution is also 0.01 M in HCl.
Let x M be the hydrogen ion concentration from ionization of acetic acid. The hydrogen ion concentration from ionization of HCl is 0.01 M. The total hydrogen ion concentration
[H+] = 0.01 + x
The acetate ion concentration is equal to the hydrogen ion concentration from ionization of acetic acid. This is also equal to the concentration of acetic acid that has dissociated.
[CH3? COO? ] = x
[CH3? COOH] = 0.05? x
Ka? = [H+] [CH3? COO? ]? / [CH3? COOH]
1.8*10?5 = (0.01+x)x / (0.05? x)? (1)
As x is very small, 0.01 + x? 0.01
0.05 – x? 0.05
Hence, the equation (i) becomes
1.8*10?5 = 0.01x? / 0.05
x = 9.0*10?5M
The degree of ionization is [CH3? COO? ]? / [CH3? COOH] = x / c?
= (9.0*10?5) /0.05?
= 1.8*10?3= 0.0018.
(b) The solution is also 0.1 M in HCl.
Let x M be the hydrogen ion concentration from ionization of acetic acid. The hydrogen ion concentration from ionization of HCl is 0.01 M. The total hydrogen ion concentration
[H+] = 0.1 + x
The acetate ion concentration is equal to the hydrogen ion concentration from ionization of acetic acid. This is also equal to the concentration of acetic acid that has dissociated.
[CH3? COO? ] = x
[CH3? COOH] = 0.05? x
Ka? = [H+] [CH3? COO? ]? / [CH3? COOH]
1.8*10?5 = (0.1+x)x? / (0.05? x) . (1)
As x is very small, 0.1 + x? 0.1
0.05? x? 0.05
Hence, the equation (i) becomes
1.8 * 10?5 = 0.1x/0.05?
x = 9.0*10?6M
The degree of ionization is [CH3? COO? ]? / [CH3? COOH] = x/c? = 9.0*10?6/ 0.05?
= 1.8 *10?4 = 0.00018.
7.54. The ionization constant of dimethylamine is 5.4 × 10–4. Calculate its degree of ionization in its 0.02M solution. What percentage of dimethylamine is ionized if the solution is also 0.1M in NaOH?
Kb= 5.4×10−4
c= 0.02M
Then, α= (Kb /c)1/2
α= (5.4×10−4 / 2 x 10-2)1/2 =0.1643
(CH3)2NH+H2O ↔ (CH3)2NH+2+OH-
[ (CH3)2NH] = 0.02 – x ≈ 0.02
[ (CH3)2NH+2] = x
[OH-] = 0.1 + x
≈ 0.1
Now, Kb= [ (CH3)2NH+2] [OH−]/ [ (CH3)2NH] = (x × 0.1) / (0.025).
x = 1.08 x 10-4
% of dimethylamine ionised = (1.08 x 10-4) x (100 / 0.02) = 0.54%
7.55. Calculate the hydrogen ion concentration in the following biological fluids whose pH are given below:
(a) Human muscle-fluid, 6.83 (b) Human stomach fluid, 1.2
(c) Human blood, 7.38 (d) Human saliva, 6.4.
a. Human muscle fluid 6.83
pH=6.83
pH=−log [H+]
∴6.83=−log [H+]
[H+]= 1.48 x 10−7M
b. Human stomach fluid, 1.2:
pH=1.2
1.2=−log [H+]
∴ [H+] = 0.063 M = 6.3 x 10-2 M
c. Human blood, 7.38:
pH=7.38=−log [H+]
∴ [H+]= 4.17 x 10−8M
d. Human saliva, 6.4:
pH=6.4
6.4=−log [H+]
[H+]= 3.98 x 10−7 M
7.57. If 0.561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH?
[KOH]= [K+]= [OH−]= (0.561×1000) / (56×200)? =0.050M
[H+]=Kw / [OH−]? =10−14 / 0.05? =2.0×10−13
pH=−log [H+]=−log (2.0×10−13)
=12.7
7.75. Assertion: The solutions of salts formed from strong acids and bases are neutral.
Reason: The cations (e.g., Na+, K+, Ca2+, Ba2+, etc.) of strong bases and anions (e.g., Cl–, Br–, NO3–, ClO4– etc.) of strong acids simply get hydrated but do not hydrolyse.
(a) The cations (e.g., Na+, K+, Ca2+, Ba2+, etc.) of strong bases and anions (e.g., Cl–, Br–, NO3–, ClO4– etc.) of strong acids simply get hydrated but do not hydrolyse, and therefore the solutions of salts formed from strong acids and bases are neutral i.e., their pH is 7. However, the other category of salts does undergo hydrolysis.
7.76. Assertion: The pH of NH4Cl solution in water is less than 7.
Reason: Ammonium hydroxide is a weak acid and remains almost unionised in solution.
(c) Ammonium hydroxide is a weak base (Kb = 1.77 * 10–5) and not a weak acid. Therefore, it remains almost unionised in solution. This results in increased of H+ ion concentration in solution making the solution acidic. Thus, the pH ofNH4Cl solution in water is less than 7.
7.77. Assertion: Higher order ionization constants (Ka2, Ka3) are higher than the lower order ionization constant (Ka1) of a polyprotic acid.
Reason: It is more difficult to remove a positively charged proton from a negative ion due to electrostatic forces.
(d) Higher order ionization constants (Ka2, Ka3) are smaller than the lower order ionization constant (Ka1) of a polyprotic acid. The reason for this is that it is more difficult to remove a positively charged proton from a negative ion due to electrostatic forces. This can be seen in the case of removing a proton from the uncharged H2CO3 as compared from a negatively charged HCO3–.Similarly, it is more difficult to remove a proton from a doubly charged HPO42– anion as compared to H2PO4–.
7.78. Assertion: Salt does not dissolve in non-polar solvent.
Reason: For non-polar solvent, solvation enthalpy is small and not sufficient to overcome lattice enthalpy of the salt.
(a) In case of a non-polar (covalent) solvent, solvation enthalpy is small and hence, not sufficient to overcome lattice enthalpy of the salt. Consequently, the salt does not dissolve in non-polar solvent. As a general rule, for a salt to be able to dissolve in a particular solvent its solvation enthalpy must be greater than its lattice enthalpy so that the latter may be overcome by former. Each salt has its characteristic solubility which depends on temperature.
MCQs:
7.79 Choose the incorrect statement.
(a) If Brönsted acid is a strong acid then its conjugate base is a weak base and vice versa.
(b) Acids are proton donors and bases are proton acceptors.
(c) higher order ionization constants (Ka2, Ka3) are higher than the lower order ionization constant (Ka1) of a polyprotic acid.
(d) All are correct.
(c) is an incorrect statement.
7.80. A catalyst will increase the rate of a chemical reaction by
(a) shifting the equilibrium to the right (b) shifting the equilibrium to the left
(c) lowering the activation energy (d) increasing the activation energy
(c) lowering the activation energy
Questions and Answers:
7.84. Why is the equilibrium constant for the reverse reaction is the inverse of the equilibrium constant for the reaction in the forwarding direction?
Equilibrium constant depends upon the way in which the reaction is written.
7.85. Does a catalyst influence the values of the equilibrium constant?
A catalyst does not affect the equilibrium composition of a reaction mixture. Catalysts influence the rate of both forward and backward reactions equally.
7.86. What is meant by equilibrium and ionic equilibrium?
Equilibrium is a state at which rate of forwarding reaction is equal to the rate of backward reaction. The equilibrium between ions and unionised molecules is called ionic equilibrium.
7.87. Define solubility product. How does common ion affect the solubility of electrolyte?
It is product of molar concentration of ion raised to the power of number of ions produced per compound in saturated solution.
Solubility of electrolyte decreases due to common ion effect.
7.88. Define Buffer Solution. Give two characteristics of a buffer solution.
The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions.
- Its pH does not change on the addition of small amount of acid or base.
- Its pH does not change on dilution or standing.
7.89. Why does the solubility of salts of weak acids like phosphates increase at lower pH?
This is because at lower pH, the concentration of the anion decreases due to its protonation. This in turn increase the solubility of the salt so that Ksp = Qsp.
7.90 A reddish colour appears on adding two drops of 0.002 M potassium thiocynate solution to 1 mL of 0.2 M iron(III) nitratesolution due to the formation of [Fe(SCN)]2+. Elaborate on the development of the reddish colour.
The intensity of the red colour becomes constant on attaining equilibrium. This equilibrium can be shifted in either forward or reverse directions depending on adding a reactant or a product. The equilibrium can be shifted in the opposite direction by adding reagents that remove Fe3+or SCN– ions. For example, oxalic acidH2C2O4), reacts with Fe3+ ions to form testable complex ion [Fe (C2O4)3]3–, thus decreasing the concentration of free Fe3+ (aq).In accordance with the Le Chatelier's principle, the concentration stress of removed Fe3+ is relieved by dissociation of [Fe (SCN)]2+ to replenish the Fe3+ions. Because the concentration of [Fe (SCN)]2+ decreases, the intensity of red colour decreases. Addition of aq. HgCl2 also decreases red colour because Hg2+ reacts with SCN– ions to form stable complex ion [Hg (SCN)4]2–.
7.91. Describe the effect of addition of inert gas on a chemical equilibrium.
If the volume is kept constant and an inert gas such as argon is added which does not take part in the reaction, the equilibrium remains undisturbed. It is because the addition of an inert gas at constant volume does not change the partial pressures or the molar concentrations of the substance involved in there action. The reaction quotient changes only if the added gas is a reactant or product involved in the reaction.
7.92. Describe the effect of change in temperature on a chemical equilibrium.
Whenever equilibrium is disturbed by change in the concentration, pressure or volume, the composition of the equilibrium mixture changes because the reaction quotient, Qc no longer equals the equilibrium constant, Kc. However, when a change in temperature occurs, the value of equilibrium constant, Kc is changed.
In general, the temperature dependence of the equilibrium constant depends on the signoff ΔH for the reaction.
- The equilibrium constant for an exothermic reaction (negative ΔH) decreases as the temperature increases.
- The equilibrium constant for an endothermic reaction (positive ΔH)increases as the temperature increases. Temperature changes affect the equilibrium constant and rates of reactions.
As the size of A increases down the group, H-A bond strength decreases and so the acid strength
(a) Increases (b) Decreases
(c) Remains constant (d) Becomes negligible
Answer: (a) increases
On addition of acetate ions to an acetic acid solution, the concentration of hydrogen ions, [H+]
(a) Becomes zero (b) Is unchanged (c) Increases (d) Decreases
Answer: (d) decreases
Equilibrium Class 11 Chemistry - FAQs
The following are the FAQs of the Equilibrium Chapter:
Commonly asked questions
What is Equilibrium in Class 11 Chemistry?
A reversible reaction where rate of the forward reaction equals to the rate of the backward reaction is called the equilibrium. It results in constant reactants and products concentrations over time.
Is class 12 chemistry equilibrium an easy chapter?
It consists of concepts such as Le Chatelier's Principle, equilibrium constant, and factors affecting equilibrium. It is not considered an easy chapter.
Which are the types of equilibrium constants?
There are four types of equilibrium constants: Formation constants, stability constants, association constants, and binding constants.
What are the conditions required for equilibrium.
There are two conditions needed for equilibrium. The net force and net torque acting upon an object must be zero.
What is the weightage of Equilibrium Class 12 Chemistry in NEET and JEE Main exam?
In NEET exam, the weightage of Equilibrium is around 3% to 6% and in JEE Main exam, the weightage is 4% to 6.6%.
Explore exams which ask questions on Chemistry Ncert Solutions Class 11th
Select your preferred stream
Chemistry Ncert Solutions Class 11th Exam
Student Forum
Other Similar chapters for you
- NCERT Chemistry 11th
- Some Basic Concepts of Chemistry
- Structure of Atoms
- Classification of Elements and Periodicity in Prop
- Chemical Bonding and Molecular Structure
- States of Matter
- Thermodynamics
- Equilibrium
- Redox Reactions
- Hydrogen
- The S-block Elements
- The p -Block Elements
- Organic Chemistry - Some Basic Principles and Tech
- Hydrocarbons
- Environmental chemistry
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test
