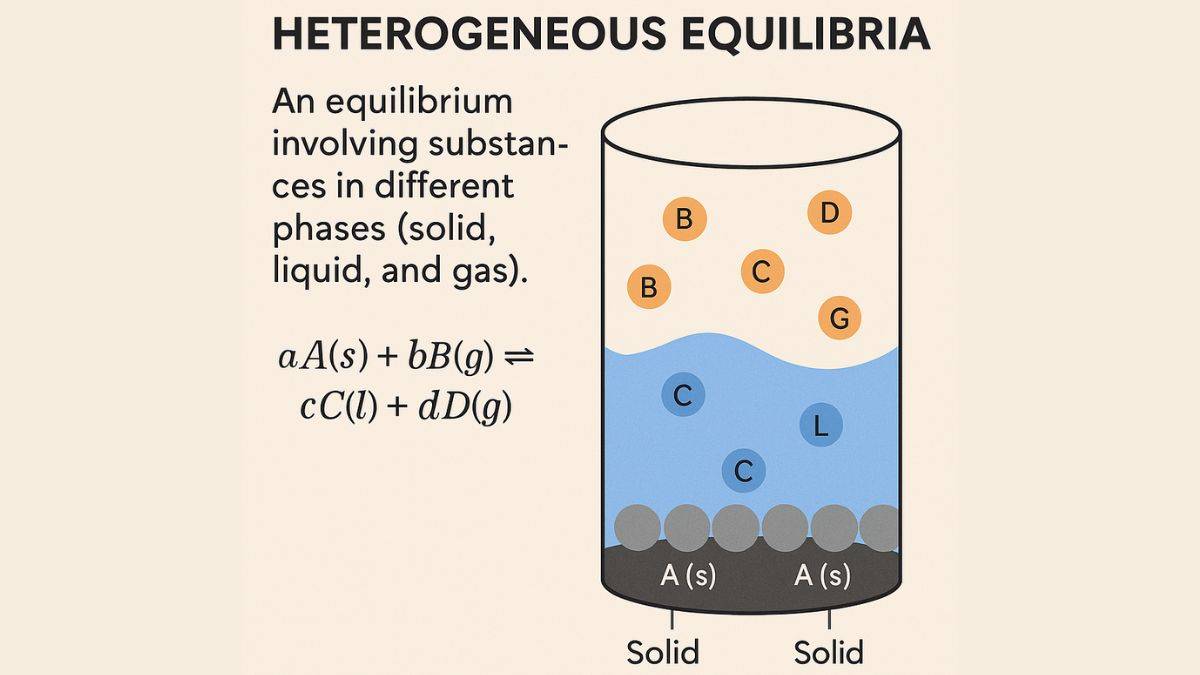
In chemical equilibrium, where reactants and products are available in different phases, such as solid, liquid, and gas, it is called heterogeneous equilibrium. This concept is applicable in the reactions involving insoluble salts, decomposition of compounds or gas-solid. In the equilibrium constant, only gaseous and aqueous solutions are considered in heterogeneous equilibrium. Knowledge of heterogeneous equilibrium will help the students know how differences in phases impact the chemical reactions and how Le Chatelier's Principle applies to multi-phase systems.
Important Links:
| NCERT Class 11 notes | |
| Chemistry Class 11 NCERT notes |
- What is Heterogeneous Equilibria?
- Equilibrium Constant in Heterogeneous Systems
- Equilibrium in Physical Change
- Factors Affecting Heterogeneous Equilibrium
- Sample JEE Main Problems
- Important Tips to Study Heterogeneous Equilibria
What is Heterogeneous Equilibria?
A state in chemical equilibrium that consists of more than one phase is called heterogeneous equilibrium. The example of a heterogeneous equilibrium is, chemical equilibrium between water vapour and liquid water in a closed container.
Also Read: NCERT Solutions | Class 11 Chemistry NCERT Solutions
Key Features of Heterogeneous Equilibria
- Equilibrium is available in two different phases
- The concentration of pure solid and liquid is not included in the expression due to constant concentration. Only gas or aqueous phases are included.
Example:
In the equilibrium expression, solid calcium carbonate and calcium oxide are excluded.
The equilibrium constant is based on CO₂ (gas).
Heterogeneous Equilibrium Key Points:
- Heterogeneous equilibrium is used in geological reactions, industrial processes, and environmental systems.
- To find the equilibrium constant, it is important to know which substance is included or excluded.
Equilibrium Constant in Heterogeneous Systems
To calculate the equilibrium constant in a heterogeneous system, the concentration or partial pressure of a gaseous or aqueous substance is required. Pure solids and liquids are excluded from the expression due to constant concentration.
General Form of the Reaction:
Equilibrium Expression:
- [A] and [C] are not included because A is solid and C is a pure liquid.
Summary:
Equilibrium in Physical Change
In chemical equilibrium, a state where two opposite physical processes occur at the same rate, with no net change in the system, is called equilibrium in physical change. It is familiar in phase changes such as melting, freezing, boiling, and evaporation.
Important Links:
| NCERT Class 12 notes | |
| Maths Class 12 Maths notes |
Example of Physical Equilibria
1. Solid ⇌ Liquid (Melting / Freezing)
Example: Ice to water: At 0°C and ice melts into water and water freezes into ice at the same rate.
2. Liquid ⇌ Gas (Evaporation / Condensation)
Example: Water ⇌ Water Vapour: Water evaporates and condenses at an equal rate in a closed container.
3. Solid ⇌ Solution ( Dissolution / Crystallization)
Example:
. Salt dissolved and crystallizes at the same rate in the saturated solution.
Factors Affecting Heterogeneous Equilibrium
Factors affecting Heterogeneous Equilibrium are:
- Temperature: The equilibrium constant is affected by the change in temperature. In an endothermic reaction, a rise in temperature favours the product. For an exothermic reaction, a rise in temperature favours the reactants.
- Pressure (for gaseous components): When the pressure is increased, it favours the side with fewer gas molecules. In case of low pressure, it will favour the side with more gas molecules.
- Concentration (for gaseous and aqueous solution): According to Le Chatelier's Principle, when we add or remove gaseous or aqueous components, the equilibrium shifts to resist the change.
Sample JEE Main Problems
Problem 1: For bar at 1000 K . Find at equilibrium.
Problem 2: For at 800 K . Initial: bar, no .Find at equilibrium.
Let .
Problem 3: For a bar at 1000 K . Convert to . bar
Problem 4: For bar at 298 K. Find the total pressure at equilibrium.
Total pressure bar.
Important Tips to Study Heterogeneous Equilibria
- Pitfall: Including solids/liquids in Tip: Omit pure phases.
- Pitfall: Miscalculating . Tip: Count only gaseous moles.
- Pitfall: Ignoring external gas effects. Tip: Use Le Chatelier’s principle.
- Tip: Practice ICE tables for gaseous equilibria.
- Tip: Memorize bar for conversions.
Chemistry Chemical Equilibrium Exam
Student Forum
Other Topics under this Chapter
- Chemical Equilibrium
- Lewis Acids and Bases
- Precipitation Titration
- Arrhenius Acid
- Hydrocyanic Acid
- Equilibrium Processes
- Equilibrium in Chemical Processes
- Homogeneous Equilibria
- Heter Heterogeneous Equilibria
- Applications of Equilibrium Constant
- Factors Affecting Equilibria
- Ionic Equilibrium in Solution
- Acids, Bases and Salts
- Ionization of Acids and Bases
- Solubility Equilibria of Sparingly Soluble Salts
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test