What is a redox reaction? The word ‘redox’ is made from the combination of (reduction-oxidation). So, a redox reaction is basically a reaction where oxidation and reduction occur at the same time. In simple terms, we can say that reactions where the transfer of electrons takes place are called redox reactions.
The Redox Reactions class 11 covers a wide range of topics in the chapter. Some topics include oxidation and reduction reactions, oxidation numbers, and redox reactions in terms of electron transfer, among others.
Through the redox reaction class 11 notes, we have detailed all the important topics of this chapter in short revision notes with examples to save your time and prepare for the class 11 chemistry exam. To help you with questions, we have Class 11 Redox Reactions NCERT Solutions as well.
- Redox Reactions – Oxidation and Reduction Reactions
- Redox Reactions in Terms of Electron Transfer Reactions
- Types of Redox Reactions
- Oxidation Number
- Redox Reactions and Electrode Processes
- Application of Redox Reactions
- Revision Notes for Class 11 Chemistry
- NCERT Solutions for Class 11 Chemistry
- About the Content Reviewer
- Redox Reaction FAQs
Redox Reactions – Oxidation and Reduction Reactions
Redox Reaction Definition-
Redox reactions are chemical reactions where oxidation and reduction occur simultaneously. The word “redox” comes from combining “reduction” and “oxidation.” These reactions are everywhere around us.
Examples of Redox Reactions:
- Reaction between Zinc and Copper Sulphate
Zn + CuSO₄ → ZnSO₄ + Cu
Oxidation: Zn → Zn²⁺ + 2e⁻
Reduction: Cu²⁺ + 2e⁻ → Cu
- Formation of Water
2H₂ + O₂ → 2H₂O
Oxidation: H₂ → 2H⁺ + 2e⁻
Reduction: O₂ + 4e⁻ → 2O²⁻
- Reaction of Iron with Chlorine
2Fe + 3Cl₂ → 2FeCl₃
Oxidation: Fe → Fe³⁺ + 3e⁻
Reduction: Cl₂ + 2e⁻ → 2Cl⁻
- Respiration (Biological Redox)
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy
Oxidation: Glucose (C atoms) oxidized to CO₂.
Reduction: O₂ reduced to H₂O
Oxidation and Reduction
Oxidation: In simple words, oxidation is the addition of oxygen or an electronegative element to a substance and the removal of hydrogen or an electropositive element from a substance.
Example of oxidation:
Addition of oxygen:
S (s) + O2 (g) → SO2 (g)
Removal of hydrogen:
Mg (s) + S (s) → MgS (s)
Reduction: In simple words, reduction is the removal of oxygen or an electropositive element from a substance and the addition of hydrogen or an electronegative element to a substance.
Examples of Reduction:
Removal of Oxygen:
2HgO(s) → 2Hg(l) + O₂(g)
Addition of Hydrogen:
CH₂=CH₂(g) + H₂(g) → CH₃-CH₃(g)
It was realised that oxidation and reduction always occur together. You cannot have one without the other. This is why these reactions are called redox reactions or oxidation-reduction reactions.
In the reaction:
2HgO(s) → 2Hg(l) + O₂(g)
- Mercury is losing oxygen
- At the same time, oxygen is being formed (which can oxidize other substances)
Redox Reactions in Terms of Electron Transfer Reactions
The modern understanding of redox reactions is based on electron transfer. It states that all types of redox reactions, including those that don’t involve oxygen.
- Oxidation: Loss of electrons
- Reduction: Gain of electrons
Any redox reaction can be split into two half-reactions:
Example: Formation of Sodium Chloride
2Na(s) + Cl₂(g) → 2NaCl(s)
Half reactions:
Oxidation half: 2Na(s) → 2Na⁺ + 2e⁻
Reduction half: Cl₂(g) + 2e⁻ → 2Cl⁻
Oxidizing and Reducing Agents
Oxidizing Agent (Oxidant): An oxidising agent accepts electrons from other substances, which causes oxidation. It reduced itself in the process.
Reducing Agent (Reductant): A reducing agent donates electrons to other substances, which causes a reduction in other substances. It oxidized itself in the process.
Example:`Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)`
- Zn loses electrons and gets oxidized; it is the reducing agent.
- Cu²⁺ gains electrons and gets reduced; it is the oxidizing agent.
Competitive Electron Transfer Reactions
Substances have different limits to lose or gain electrons. This creates competition for electrons.
Understand from the Experiment:
Place a zinc strip in a copper sulfate solution:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Observations:
- The zinc strip gets coated with copper
- The blue color of the solution fades
- Heat is evolved
Electron Transfer:
- Zn loses electrons: `Zn(s) → Zn²⁺(aq) + 2e⁻`
- Cu²⁺ gains electrons: `Cu²⁺(aq) + 2e⁻ → Cu(s)`
Based on electron transfer tendency, metals can be arranged in an activity series:
Decreasing Order of Reactivity:
K > Ca > Na > Mg > Al > Zn > Fe > Ni > Pb > H > Cu > Ag > Au
Types of Redox Reactions
There are four types of redox reactions:
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Disproportionation Reactions
Combination Reactions
Reactions in which elements combine to form compounds are called combination reactions. For example- C(s) + O₂(g) → CO₂(g)
Decomposition Reactions
Reactions in which compounds break down into elements are called decomposition reactions. They are the opposite of a combination reaction. For example- 2H₂O(l) → 2H₂(g) + O₂(g)
Displacement Reactions
Reactions in which one element displaces another are called displacement Reactions. For example- Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Metal Displacement:
- More reactive metals displace less reactive ones
- Used in metallurgy for metal extraction
Non-metal Displacement:
- Hydrogen displacement from water and acids
- Halogen displacement reactions
Disproportionation Reactions
Reactions in which the same element undergoes both oxidation and reduction are called disproportionation reactions. For example- Cl₂(g) + 2OH⁻(aq) → ClO⁻(aq) + Cl⁻(aq) + H₂O(l)
Chlorine: 0 → +1 (oxidation) and 0 → -1 (reduction)
Oxidation Number
Oxidation number or oxidation state shows the number of electrons gained or lost by an atom. It’s a method to track electrons in compounds.
Rules for Assigning Oxidation Numbers:
Rule 1: The oxidation number of atoms in free elements is zero.
Examples: Na, H₂, O₂, Cl₂, P₄, S₈ all have oxidation number 0
Rule 2: For monatomic ions, oxidation number equals the charge on the ion.
Examples: Na⁺ = +1, Cl⁻ = -1, Al³⁺ = +3, O²⁻ = -2
Rule 3: In compounds: Group 1 elements: +1, Group 2 elements: +2, and Aluminum: +3
Rule 4: Hydrogen is usually +1, except in metal hydrides, where it’s -1.
Examples: H₂O (+1), HCl (+1), but NaH (-1), CaH₂ (-1)
Rule 5: Oxygen is normally -2, with exceptions:
- In peroxides like H₂O₂: -1
- In superoxides like KO₂: -½
- When bonded to fluorine: positive values
Rule 6: Fluorine is always -1 in compounds.
Rule 7: Other halogens are usually -1, except when combined with oxygen.
Rule 8: The sum of oxidation numbers
- In neutral compounds = 0
- In polyatomic ions = the charge on the ion
Examples of Oxidation Number Calculation
H₂SO₄
- H: +1 each, total = +2
- O: -2 each, total = -8
- For neutral compound: S + 2 - 8 = 0
- Therefore: S = +6
Stock Notation
This notation uses Roman numbers to show oxidation state or oxidation number.
FeO → Fe(II)O
Fe₂O₃ → Fe₂(III)O₃
CuCl → Cu(I)Cl
CuCl₂ → Cu(II)Cl₂
Balancing Redox Reaction
To balance a redox equation, two methods can be followed, which are the oxidation number method and the half-reaction method.
Oxidation Number Method
To write a balanced equation of the oxidation-reduction reaction or redox reaction through the oxidation number method, follow these steps.
- Write correct formulas for reactants and products
- Assign oxidation numbers to all elements
- Identify which elements change oxidation numbers
- Balance the atoms whose oxidation numbers change
- Balance charge by adding H⁺ or OH⁻
- Balance hydrogen and oxygen by adding H₂O
Half Reaction Method
In this method, you balance each half of the equation at a time, then combine both. Here are the steps.
- Write separate oxidation and reduction half reactions
- Balance atoms other than H and O
- Balance oxygen by adding H₂O
- Balance hydrogen by adding H⁺
- Balance the charge by adding electrons
- Make electrons equal in both half reactions
- Add half reactions and cancel electrons
Redox Reactions as the Basis for Titrations
Redox titrations are analytical methods used to determine the concentration of oxidizing or reducing agents in solution., Redox titrations use various methods to detect when the reaction is complete.
Types of Redox Indicators
Self-Indicators
Some reagents act as their own indicators due to their intense color.
Potassium Permanganate (KMnO₄):
- Deep purple color in the solution
- Becomes colorless when reduced to Mn²⁺
- The first permanent pink color indicates the endpoint
- Can detect concentrations as low as 10⁻⁶ mol L⁻¹
External Indicators
Starch-Iodine System:
Many oxidizing agents can oxidize iodide ions to iodine:
The liberated iodine gives an intense blue color with starch. This iodine is then titrated with sodium thiosulfate:
The endpoint is reached when the blue color disappears.
Diphenylamine Indicator:
Used with dichromate titrations, gets oxidized after the equivalence point and produces an intense blue color.
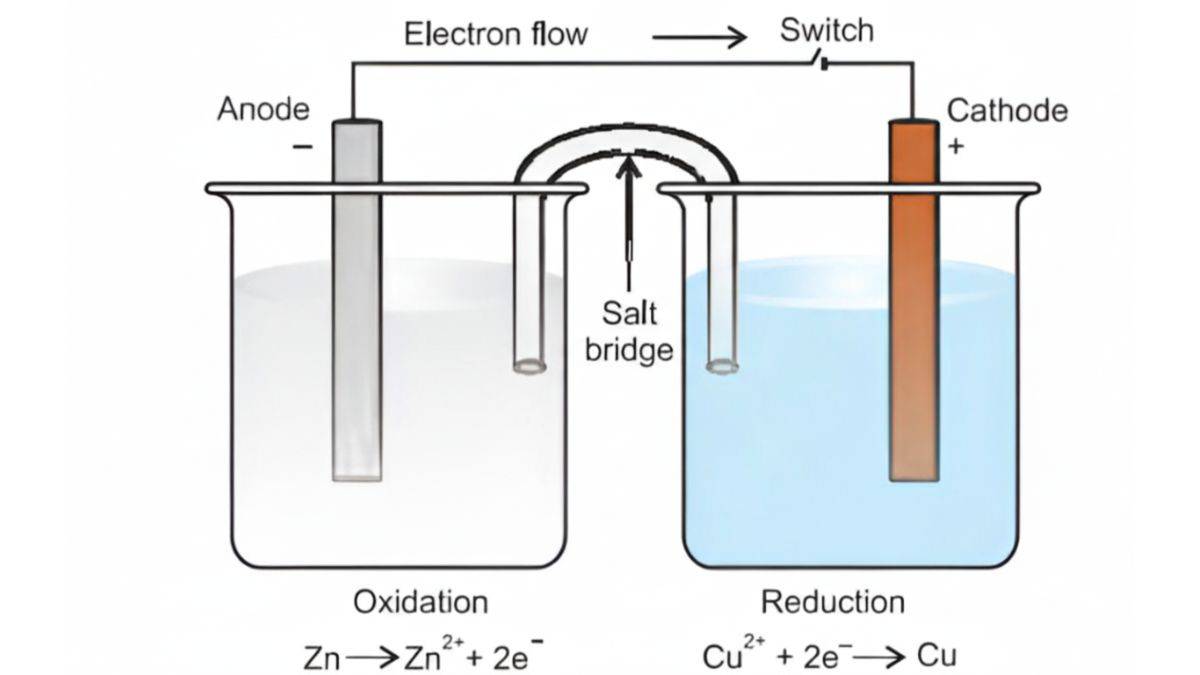
Redox Reactions and Electrode Processes
Electrode Processes
When a redox reaction occurs in an electrochemical cell, it can produce electricity. This connection results in the formation of the basis of batteries, fuel cells, and electrolysis.
The Daniell Cell
With the aid of the Daniell cell, you can see how redox reactions can produce electricity with the aid of zinc and copper. First, you place a zinc electrode in a ZnSO₄ solution and a copper electrode in a CuSO₄ solution. Use a salt bridge to link the solutions and an external wire to link the electrodes.
Reactions:
At zinc electrode: Zn(s) → Zn²⁺(aq) + 2e⁻ (oxidation)
At copper electrode: Cu²⁺(aq) + 2e⁻ → Cu(s) (reduction)
Overall: Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
Electrode Terminology
Anode, the electrode where oxidation takes place or electrons are released. And Cathode, the electrode where reduction takes place or electrons are used.
Redox Couples
A redox couple represents the oxidized and reduced forms of a substance that takes part in an oxidation or reduction half-reaction.
It is written as: Oxidized form/Reduced form
For Example,
- Zn²⁺/Zn,
- Cu²⁺/Cu
- Fe³⁺/Fe²⁺
Standard Electrode Potentials
Each redox couple has a characteristic electrode potential (E°) measured under standard conditions, which are:
- 25°C temperature
- 1 M concentration
- 1 bar pressure
Application of Redox Reactions
Redox reactions are applied in different industries and in daily life.
Redox Reaction- Industrial Applications
- Metallurgy- Redox reactions are fundamental to metal extraction and purification:
Extraction of Iron:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
Aluminum Production
2Al₂O₃ + 3C → 4Al + 3CO₂
Copper Refining:
Electrolytic purification using redox reactions at electrodes.
- Chemical Manufacturing
Production of Chlorine and Sodium Hydroxide:
2NaCl + 2H₂O → Cl₂ + 2NaOH + H₂
Manufacture of Hydrogen Peroxide:
H₂SO₄ + BaO₂ → BaSO₄ + H₂O₂
Redox Reaction- Biological Applications
- Cellular Respiration- The process by which cells extract energy from glucose:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy
- Photosynthesis- Plants convert light energy into chemical energy:
6CO₂ + 6H₂O + light → C₆H₁₂O₆ + 6O₂
- Water is oxidized to oxygen
- Carbon dioxide is reduced to glucose
- Light energy drives the process
Importance of Redox Reaction in Daily Life
Redox reactions are involved in our daily life activities
- Cooking (browning, caramelization)
- Cleaning (bleaching, disinfection)
- Medicine (antioxidants, drug metabolism)
- Transportation (fuel combustion, battery operation)
- Communication (battery-powered devices)
For a detailed chapter, you can check Class 11 Redox Reaction NCERT PDF.
Revision Notes for Class 11 Chemistry
Here you can check the Class 11 chemistry notes for other chapters:
| Thermodynamics Class 11 Notes |
|
| Classification of Elements and Periodicity in Properties Class 11 Notes |
|
| Hydrogen Class 11 Notes |
|
| States of Matter Class 11 Notes |
The s-Block Element Class 11 Notes |
| Organic Chemistry – Some Basic Principles and Techniques Class 11 Notes |
The p-Block Element Class 11 Notes |
| Environmental Chemistry Class 11 Notes |
NCERT Solutions for Class 11 Chemistry
About the Content Reviewer
Redox Reaction FAQs
Commonly asked questions
What are all the redox reactions?
Redox reactions are of four kinds: Decomposition, Combination, Displacement, and disproportionation reactions.
What is the redox reaction formula?
Redox reaction does not have a fixed formula but is an equation that forms when oxidation and reduction occur simultaneously. For example, 2Fe + 3Cl? 2FeCl? (Fe loses electrons and Cl gains).
What is involved in an oxidation-reduction reaction?
In an oxidation-reduction reaction or redox reaction, the process of oxidation (addition of electrons) and reduction (removal of electrons) happens at the same time.
Chemistry Redox Reactions Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics
Popular Courses After 12th
Exams accepted
CA FoundationExams accepted
ICSI ExamExams accepted
BHU UET | GLAET | GD Goenka TestBachelor of Business Administration & Bachelor of Law
Exams accepted
CLAT | LSAT India | AIBEExams accepted
IPMAT | NMIMS - NPAT | SET
Exams accepted
BHU UET | KUK Entrance Exam | JMI Entrance ExamBachelor of Design in Animation (BDes)
Exams accepted
UCEED | NIFT Entrance Exam | NID Entrance ExamBA LLB (Bachelor of Arts + Bachelor of Laws)
Exams accepted
CLAT | AILET | LSAT IndiaBachelor of Journalism & Mass Communication (BJMC)
Exams accepted
LUACMAT | SRMHCAT | GD Goenka Test