We have provided the previous year JEE Advanced Chemistry question paper. Also, you will find the 14 most difficult questions asked in the past JEE Advanced exam. Check the complete article.
JEE Advanced Chemistry Questions: Chemistry is consider to be most easy paper in JEE Advanced. But sometime tricky questions are asked which might be time consuming. Majority of question in JEE Advanced chemistry paper is based on p-block, Chemical bonding, Coordination compounds, Electrochemistry, Ionic & Chemical Equilibrium, Electrochemistry & Surface Chemistry, Aldehyde & Ketones, Polymers, and Biomolecules.
To master the question based on these topic intense practice is required. Students can solve previous year question for this. In this article we have provided past year JEE Advanced Chemistry questions. Also some tricky questions are provided here.
- 15 Most Difficult JEE Advanced Chemistry Questions Asked in Previous 10 Years
- All JEE Advanced Chemistry Questions From Previous Years
- JEE Advanced Chemistry Section Overview
15 Most Difficult JEE Advanced Chemistry Questions Asked in Previous 10 Years
Check below the most difficult questions in JEE Advanced chemistry in the past 10 years as analysed by the experts:
Q1: The option(s) with only amphoteric oxides is (are) (JEE Advanced 2020)
- NO, B2O, PbO, SnO2
- Cr2O3, CrO, SnO, PbO
- Cr2O3, BeO, SnO, SnO2
- ZnO, Al2O3, PbO, PbO2
Ans: C and D
Q2: The 1st, 2nd and 3rd ionization enthalpies I1, I2 and I3, of four atoms with atomic numbers n, n + 1, n + 2 and n + 3 where n < 10 are tabulated below. What is the value of n? (JEE Advanced 2020)
| Atomic Number | Ionization Enthalpies (kJ/mol) | ||
|---|---|---|---|
| I1 | I2 | I3 | |
| n | 1681 | 3374 | 6050 |
| n+1 | 2081 | 3952 | 6122 |
| n+2 | 496 | 4562 | 6910 |
| n+3 | 738 | 1451 | 7733 |
Ans: n=9
Q3: Identify the least stable ion among the following: (JEE Advanced 2022)
- Li-
- Be-
- B-
- C-
Ans: B
Q4: identify the correct order of acidic strength of CO2, CuO, CaO, H2O (JEE Advanced 2022)
- CaO < CuO < H2O < CO2
- H2O < CuO < CaO < CO2
- CaO < H2O < CuO < CO2
- H2O < CO2 < CaO < CuO
Ans: A
Q5: Among the following, the number of elements showing only one non-zero oxidation state is: (JEE Advanced 2010)
O, Cl, F, N, P, Sn, Tl, Na, Ti
Ans: 2
Q6: Assuming 2s - 2p mixing is not operative, the paramagnetic species among the following is: (JEE Advanced 2014)
- Be2
- B2
- C2
- N2
Ans: C
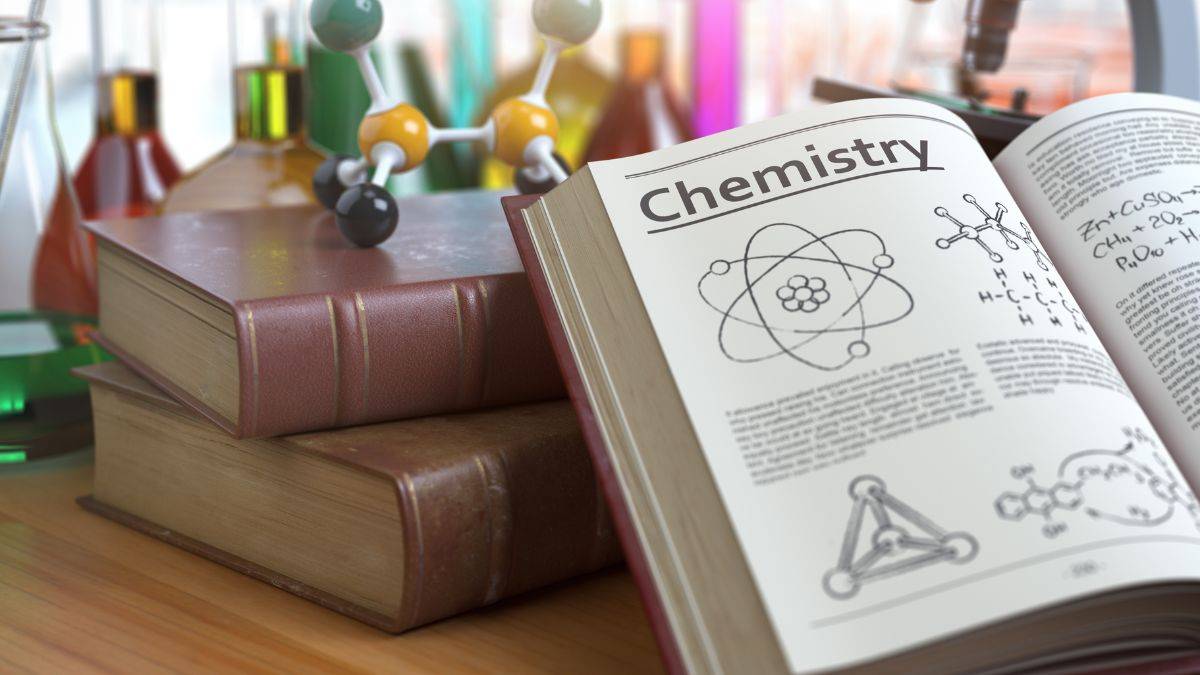
Q7: If the distribution of the molecular speed of a gas is as per the figure shown below, then the ratio of the most probable, the average and the root mean square speeds, respectively, is: (JEE Advanced 2020)
- 1 : 1 : 1
- 1 : 1 : 1.224
- 1 : 1.128 : 1.224
- 1 : 1.128 : 1
Ans: B
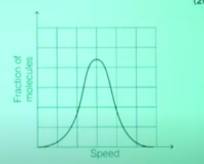
Q8: 1 mol of a monoatomic real gas satisfies the equation p(V-b) = RT where, b is a constant. The relationship of interatomic potential V(r) and interatomic distance for as is given by: (JEE Advanced 2015)
Ans: C
Q9: The treatment of an aqueous solution of 3.74 g of Cu(NO3)2 with excess Kl results in a brown solution along with the formation of a precipitate. Passing H2S through this brown solution gives another precipitate X. The amount of X (in g) is ______________ ? (JEE Advanced 2022, paper 1)
[Given: Atomic mass of H = 1, N=14, O = 16, S = 32, K = 39, Cu = 63, I = 127]
Ans: 0.32
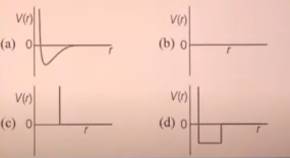
Q10: For the given close packed structure of a salt made of cation X and anion Y shown below (ions of only one face are shown for clarity), the packing fraction is approximately: (JEE Advanced 2021, paper 1)
(packing fraction = packing efficiency ÷ 100)
Ans: 0.63
Q11: The boiling point of water in a 0.1 molal silver nitrate solution (solution A) is x°C. To this solution A, an equal volume of 0.1 molal aqueous barium chloride solution is added to make a new solution B. The difference in the boiling points of water in the two solutions A and B is y x 10-2 °C.
(Assume: Densities of the solutions A and B are the same as that of water and the soluble salts dissociate completely. Use: Molal elevation constant (Ebullioscopic Constant), K = 0.5 K kg mol-1; Boiling point of pure water as 100°C).
The value of lyl is__________ (JEE Advanced 2021, paper 1)
Ans: 2.5
Q12: In a bimolecular reaction, the steric factor P was experimentally determined to be 4.5. The correct option(s) among the following is(are) (JEE Advanced 2017, paper 2)
- The activation energy of the reaction is unaffected by the value of the steric factor
- Experimentally determined value of frequency factor is higher than that predicted by Arrhenius equation
- Since P= 4.5, the reaction will not proceed unless an effective catalyst is used
- The value of the frequency factor predicted by the Arrhenius equation is higher than that determined experimentally
Ans: A and B
Q13: The decomposition reaction 2N2O5 (g) → 2N2O4 (g) + O2(g) is started in a closed cylinder under Isothermal isochoric condition at an initial pressure of 1 atm. After Y x 103 s, the pressure inside the cylinder is found to be 1.45 atm. If the rate constant of the reaction is 5 x 10-4 S-1, assuming ideal gas behaviour, the value of Y is___________ (JEE Advanced 2019, paper 2)
Ans: 2.30
Q14: Consider a Helium (He) atom that absorbs a photon of wavelength 330 nm. The change in velocity (in cm s-1) of the He atom after the photon absorption is ______________
(Assume momentum is conserved when photon is absorbed. Use Planck constant = 6.5 X 10-13 J s, Avogadro number = 6 X 1023 mol-1, Molar mass of He = 4 m mol-1) (JEE Advanced 2021)
Ans: 30 cm/s
Q15: The atomic masses of He and Ne are 4 and 20 a.m.u., respectively. The value of the de Broglie wavelength of He gas at -73°C is "M" times that of the de Broglie wavelength of Ne at 727°C. M is - (JEE Advanced 2013)
Ans: 5
Also Read:
You can prepare for all entrance tests at same time. It is a tough task but not impossible. For this a proper strategy is required. Here are some important tips.
- Compare the syllabus: All engineering entrance exams have almost the same syllabus. There might be some additional topics in some exams. The aspirant must study the syllabus for all exams and find out the topics that are extra, and prepare those separately. Almost 90% of the topics will be common for all entrance exams, and hence, syllabus completion for one exam will lead to completion of the syllabus for all the exams.
- Prepare for JEE Mains and incorporate the preparation for all other exams: Apart from JEE Advanced, all the exams are pretty straightforward for a well-prepared candidate. JEE Advanced require a higher level of preparation as the questions are multi-conceptual. However, the state-level entrance exams, and exams like BITSAT, VITEEE, etc., have direct questions. Most of the questions in JEE Mains are also direct and require the application of basic concepts. Hence, the candidates must prepare for one exam, like JEE Mains, that will cover their preparation for all other exams.
- JEE Advanced needs extra effort: For JEE Advanced candidates must focus on time management and building exam day temperament. Learn how to apply multiple concepts to a question.
The bottom line is to prepare for JEE Mains to cover the preparation for all other CETs and exams like BITSAT and VITEEE. For JEE Advanced, work on sharpening your skills of time management, stress handling, multiple concept application, speed and accuracy.
So far no student has ever scored full marks in JEE Advanced signifies exam's difficulty level. Highest marks one has achieved to date in JEE Advanced are 355 marks out of 360. Ved Lahoti scored these marks in 2024.
All JEE Advanced Chemistry Questions From Previous Years
Above we curated and provided you the most difficult questions in JEE Advanced Chemistry over the past 10 years. However, candidates who wish to have all the previous year JEE Advanced Chemistry questions for practice, can download the PDF files below:
JEE Advanced Chemistry Questions with Solutions 2024 to 2019:
Check below the JEE Advanced Chemistry questions with solutions PDF:
| Year |
JEE Advanced Chemistry questions with solutions |
|---|---|
| 2024 |
Paper 1 - Download here Paper 2 - Download here |
| 2023 |
Paper 1 - Download here Paper 2 - Download here |
| 2022 |
Paper 1 - Download here Paper 2 - Download here |
| 2021 |
Paper 1 - Download here Paper 2 - Download here |
| 2020 |
Paper 1 - Download here Paper 2 - Download here |
| 2019 |
Paper 1 - Download here Paper 2 - Download here |
Also Check:
| JEE Advanced Physics Questions | JEE Advanced Maths Questions |

JEE Advanced Chemistry Section Overview
Check below the details regarding the JEE Advanced Chemistry section in JEE Advanced question paper followed in the past 2 years:
Total Marks in JEE Advanced Chemistry section: 120
- 60 marks in JEE Advanced paper 1
- 60 marks in JEE Advanced paper 2
In each paper of JEE Advanced, the Chemistry section has the following sections:
- Sec-I (Max. marks-12)
- Sec-II (Max. Marks-12)
- Sec-III (Max. marks-24)
- Sec-IV (Max. marks-12)
Number of questions and marks distribution in each of the sections is as follows:
| Section |
Number of questions and total marks |
Marking Scheme |
|---|---|---|
| 1 |
4 questions of 12 marks |
|
| 2 |
3 questions of 12 marks |
|
| 3 |
6 questions of 24 marks |
|
| 4 |
4 questions of 12 marks |
|
Read More:

Currently - Assistant Manager, Content
Role - News and Feature Writer, Content Editor
Expertise - Engineering Education and Counselling
Mamona Majumder specialises in news and feature writing, and content editing. W
Read Full BioNews & Updates
Explore Other Exams
27 Feb '26 - 28 Feb '26 | JEE Main 2026 session 2 form c... |
Mar '26 | JEE Main 2026 Admit Card Sessi... |
3 Nov '25 - 16 Apr '26 | SRMJEEE 2026 Registration (Pha... |
3 Nov '25 - 4 Jun '26 | SRMJEEE 2026 Registration (Pha... |
3 Feb '26 - 16 Mar '26 | COMEDK Application Form 2026 |
10 Apr '26 - 13 Apr '26 | COMEDK Form Correction 2026 |
1 Oct '25 - 15 Mar '26 | MET 2026 application form - Ph... |
31 Mar '26 - 2 Apr '26 | MET 2026 Slot Booking Phase 1 |
15 Dec '25 - 16 Mar '26 | BITSAT 2026 Application Form S... |
18 Mar '26 - 20 Mar '26 | BITSAT 2026 Form Correction Se... |
19 Feb '26 - 4 Apr '26 | TS EAMCET Application Form 202... |
7 Apr '26 - 8 Apr '26 | TS EAMCET 2026 form correction |
Student Forum
Answered 4 days ago
IIT Jammu accepts the JEE Advanced entrance exam for BTech admission. For the students belonging to the General AI quota, the cutoff ranks ranged between 5988 and 18156 for BTech admission across multiple specialisations. For the category-wise cutoff range, refer to the table below:
| Category | JEE Advanced Cutoff Range 2025 |
|---|---|
| Genenral | 5988 - 18156 |
| OBC | 2594 - 6153 |
| SC | 1266 - 3346 |
| ST | 763 - 1555 |
| EWS | 811- 2665 |
R
Contributor-Level 10
Answered 4 days ago
For IIT Palakkad B.Tech. admission, the JEE Advanced closing ranks ranged between 2229 and 5841 for the students belonging to the OBC category. Candidates can refer to the table below to view the JEE Advanced round-wise cutoff range for 2025.
| Round | JEE Advanced Cutoff Range 2025 |
|---|---|
| 1 | 2229 - 5167 |
| 2 | 2293 - 5445 |
| 3 | 1153 - 2990 |
| 4 | 1168 - 2990 |
| 5 | 1182 - 3024 |
R
Contributor-Level 10
Answered 4 days ago
Yes, if you belong to the General AI category, you can get admission to CSE at IIT Tirupati with a JEE Advanced cutoff rank of 4000. The cutoff rank for admission varies across different categories and rounds. In 2025, for CSE admission, the last round cutoff ranks varied between 4620 and 5034 for t
R
Contributor-Level 10
Answered 4 days ago
For IIT Tirupati, the JEE Advanced last round closing ranks ranged between 5034 and 16548 for BTech admission in the General category under the AI quota. Likewise, the cutoff range varies across different categories and rounds. For the last round, the category-wise range is given below:
| Category | Last Round Cutoff 2025 |
|---|---|
| General | 5034 - 16548 |
| OBC | 2113 - 5522 |
| SC | 1018 - 3091 |
| ST | 553 - 1376 |
| EWS | 733 - 2434 |
| PWD | 167 - 228 |
R
Contributor-Level 10
Answered a week ago
Candidates must be very careful in filling JEE Advanced application form as the correction facility is not provided post the last date of registration. It is thus advised that candidates verify all the details they fill in the form at least once and make the corrections before the last date to apply
S
Contributor-Level 10
Answered a week ago
If the PwD certificate is valid and issued by the competent authority, it will be considered for availing the reservation. However, it is advsied to get the UDID card before admission process.
S
Contributor-Level 10
Answered a week ago
Candidates with benchmark disabilities are eligible to avail scribe facility. For this, they have to first mark their PwBD status/category in application form. Candidates can bring their own scribe or can take the facility from the organizing institute.
S
Contributor-Level 10
Answered a week ago
The procedure to apply for IIT JEE Adv includes four main steps
- Login through JEE Main application number and date of birth
- Fillling of required details
- Uploading documents
- Payment of application fee
S
Contributor-Level 10
Answered a week ago
In case of failed JEE Advanced application fee payment, the money deited from account will be credited back to the account within 24-hours. In case the money is not recieved back, they can contact the JEE Adv official through email or phone.
S
Contributor-Level 10
Answered a week ago
Candidates who are appearing in their class 12 exam in 2026 need not provide their mark details while filling the JEE advanced application form. They just need to fill their year of passing class 12 year as 2026. However, they must ensure to score minimum 75% marks in order to be eligibile for admis
S
Contributor-Level 10
 Registration - 6 Apr '26 - 2 May '26
Registration - 6 Apr '26 - 2 May '26



Preparation for JEE Advanced begins by knowing exam syllabus. Every student must follow syllabus. Once preparation is done then start solving previous year questions for self-assessment. This will give an overview of your preparation.